As is often the case in science, a remarkable discovery by Monash University’s regenerative medicine experts happened completely by accident. Or, more accurately, it was a discovery born from a mistake.
Researchers at ARMI, the University’s Australian Regenerative Medicine Institute, were looking into unanswered questions regarding how muscle cells respond to stem cells when the muscle is damaged. The point of this was to figure out a way of getting muscle to better regenerate, or rebuild itself.

The ARMI team was led by then PhD student Dr Dhanushika Ratnayake and ARMI’s director of research, Professor Peter Currie. They had been making images and “home movies” of goings-on inside the larvae of the institute’s zebrafish larvae, the go-to tool for studying regeneration, because the tiny baby fish are incredibly adept at rebuilding themselves.
“One of these movies,” says Professor Currie, “captured a bizarre behaviour where the muscle cells came out to guide the stem cells back to where the hole was, the wound.”
He was convinced this was due to the actions of a macrophage, a type of cell that helps the immune system. But he was mistaken.
“Danni proved within a month that the boss was completely wrong,” he says.
A medical science breakthrough
The answer was with the macrophages – but it was something new to medical science.
The landmark project of experiments – over five years – is published in Nature journal this week, with collaborators from the Netherlands, France, Germany, the University of Melbourne, and the Walter and Eliza Hall Institute of Medical Research (WEHI).
At ARMI, Associate Professor Mikael Martino and Professor Graham Lieschke’s linked labs worked closely on the project.
Dr Ratnayake is lead author. She takes up the story.
“We saw that these particular macrophages, or immune cells, came to the injury site and then, to our surprise, hung around,” she says. “We thought they came in and cleaned up the mess and left; we thought that was their role.
“But we saw some stayed with the stem cells, and had very intimate associations with the stem cells, always in contact. We saw that this subset of macrophages are the ones that make the muscle stem cells proliferate, and this proliferation is essential for them to make new muscle fibre.”
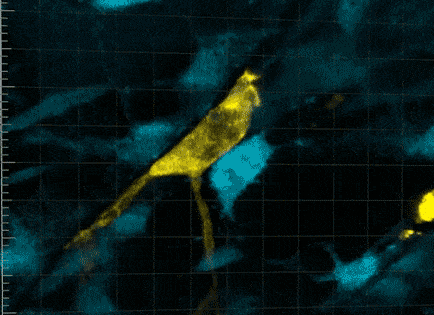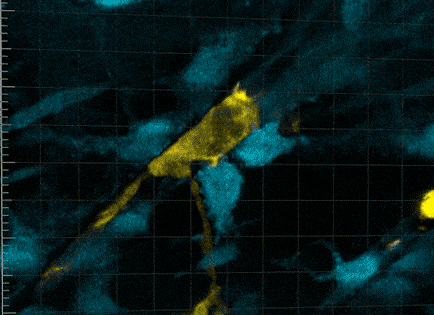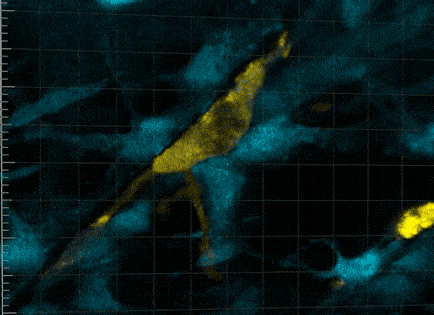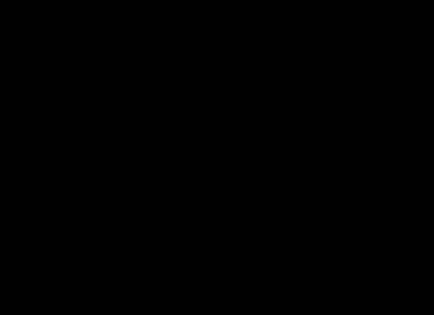
So it wasn’t that the macrophages were guiding the stem cells towards a wound, but, in effect, grabbing it. It was that the macrophages were communing with the stem cells to trigger cellular activity that hasn’t been seen before.
“This was very strange for us to see,” says Professor Currie. “We found that no stem cell division occurred without a prolonged period of this intimate relationship with the macrophage, for more than five hours.
“The macrophage is physically cuddling the stem cell. Beautiful tendrils of membrane encompassing and overlapping in waves of protrusion.”
Read more: Zebrafish and their leading role in regeneration research
Upon further detailed inspection via molecular profiling, the team found that a specific molecule secreted from a specific macrophage stimulated muscle cell repair in both zebrafish and other pre-clinical models. This molecule is named nicotinamide phosphoribosyltransferase, or NAMPT.
“This is perhaps the discovery with the most therapeutic potential we’ve ever made,” Professor Currie says. “There are massive areas that are central to the human condition.”
The first is for genetic muscle disease such as muscular dystrophies, for which at the moment there’s no therapy except a standard of care.
“Once you lose muscle mass, the therapies aren’t geared towards replacing it; they’re geared towards stopping it happening further. So we could try to reverse the process.”

Similar to cancer treatment
There’s a potential parallel to cancer treatment that already uses cellular therapies.
“Can the pro-regenerative macrophages be harnessed to accelerate regeneration as a cellular therapy? Is there a link to regeneration? Would the macrophage be the right kind of cell? If so, can we get the right macrophages to the right place at the right time?”
There are muscle injuries from trauma, sport, invasive surgery or war. And then there’s ageing, which Professor Currie calls a more “speculative” field, but one that could clearly benefit.
“Sadly, every single person who lives to a certain age will become frail. It’s inevitable. Largely this is due to loss of bone and muscle. Muscle frailty is only in part due to stem cells; there are other systemic failures that happen during ageing. But increasing muscle mass by stimulating stem cells during ageing may reverse aspects of muscle loss.”
The project is funded to continue for the next five years. “We think all this new information could be very useful,” he says. “Now we have to prove that it can work in people.”
Waking up the stem cells
Muscles have the second-oldest known stem cell in the human and vertebrate body. It’s called a satellite cell, and is a “tissue resident stem cell”. It’s asleep most of the time, until muscle is injured, and it only does this one job.
But triggering it has, until now, been the problem – “these signals and how they’re regulated are the holy grail in terms of activating stem cells”, Professor Currie says. Once activated, they grow and replicate.
The zebrafish and their larvae are such an important model for ARMI because they’re transparent, and the muscle system is the same as humans. So it was a case of lighting up the cellular components inside and having a good look. This is why most of the landmark Nature paper is largely about the imaging technology (multi-cellular imaging) that was used.
Professor Currie says Dr Ratnayake has been a “spectacular, totally mind-blowing, highly intelligent PhD student. You could never give her enough kudos for this work.”
She’s taken up a post-doctoral role in the Netherlands.
“I was given free rein on this,” she says, “which was challenging at first, but became liberating. Peter was there to give me a nudge in the right direction, but he gave me freedom and access to resources.
“We were only limited by our imagination.”





