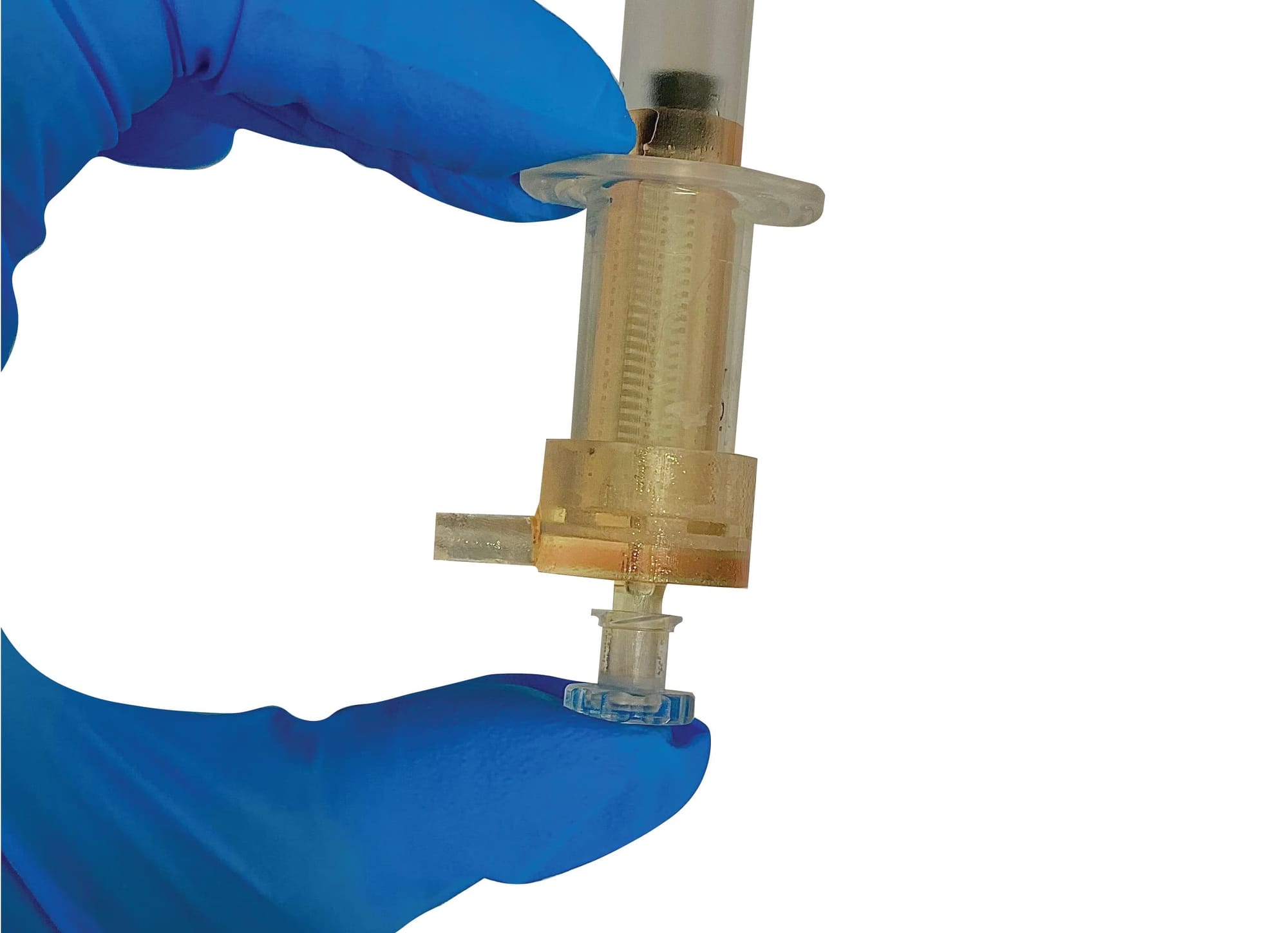
The research team behind a promising new fertility tool called the “sperm syringe” spells out the “global health concern” in reproduction in no uncertain terms.
The Monash-led team is published in Advanced Technology Materials; the sperm syringe paper graces the journal’s cover.
Infertility, the authors write, affects “… one in six couples in Australia, and as high as 70 million couples worldwide. Male factors, including low sperm count, lack of viability, poor motility, abnormal morphology, and/or low DNA integrity contribute to a total of 45% of infertility cases.”
“Motility” here means sperm’s ability to move from one point to another – essential to human reproduction. “Morphology” means size and shape.
The sperm syringe has been built to filter the best bits for IVF treatments – the bits that are healthy and can move well.
Dr Reza Nosrati, who supervised the work, is the Director of the Applied Microfluidics and Bioengineering (AMB) Lab in the Department of Mechanical and Aerospace Engineering. He explains the device to Lens.
It looks a lot like a normal syringe. How does it work?
It’s a very sophisticated filter with a very specific pore size and very specific length that the cells need to swim through to get from one side, which is the raw sample of semen, to the other side, which is the selected population.
It has 560 microchannels in the filter, which act as a sorting platform for sperm based on its boundary-following behaviour.
The highly motile cells swim through this network of microchannels connected to a central collection chamber. Then, after usually 15 to 20 minutes, you have a lot of motile cells concentrating within the central port, and then it’s possible to push those selected cells out.
It sounds like a relatively simple design?
It’s a very simple device, and I think that's one of the key advantages.
We want it to be simple, because when it comes to microfluidics, sperm selection and infertility, you have a lot of technology doing a brilliant job, but they don’t get translated necessarily, because they’re just too complicated to be operated in a clinical setting.
And the process with the sperm is reasonably simple, too?
Yes, if you look at natural reproduction, the sperm is released, and it has to go somewhere, right? It’s released, say, 20 centimetres from the egg, and it needs to go through all different types of races to get to the egg – through flow environment, chemical signals, temperature gradient, changing viscosity, and all of those.
What we do is take one part of that selection process, which happens naturally in vivo, and then we put it in a simple device and reduce that selection length from that centimetre scale into just a few millimetres.
Then we make it simpler to filter the cells, but we’re still using the same natural mechanism.
The key thing is you’re designing the syringe for clinical use, not in labs.
Yes, and I think we show that we can do a better sperm selection compared to existing clinical methods, we get a better number of cells, we get good volume.
We have previous technologies that were also trying to select sperm, and we’ve been trying to push them for commercialisation as well, but one of the challenges with those developed by my group – and also, I think, most of microfluidic groups around the world – is the fact that the volumes they’re collecting from those devices are not as high, which makes it more challenging for clinicians to actually use the device.
We wanted something to give a good number of cells, a good volume of cells, and in a process that’s not too complicated, that’s relatively robust – then the clinicians can easily use the device.
Read more: Going swimmingly: Sperm breakthrough offers new fertility hope
What are you doing with it in terms of improvements?
We still need to improve the user interface. We’ve also switched from the rapid 3D printing prototyping approach that we’ve used to develop and test the device, to something that’s more scalable, such as potentially fabricating and manufacturing this device through injection moulding, which is what most clinical consumables are made of.
We now have essentially the second version of the device, which is more or less similar in geometry and function to the original version, but the way that we assemble the device and put it together is in a way that is much easier to mass-manufacture at the really low cost.
We’ve tested the second version of the device in the lab, and we know it works relatively the same as the original version.
Now we need to just find the required support to get the mass-manufacturing going, produce it from the material that we can do clinical testing with, and then make sure that, basically, the sperm that we’re selecting are of high quality, and actually can improve the fertilisation rate, and be safe for both egg and embryo.
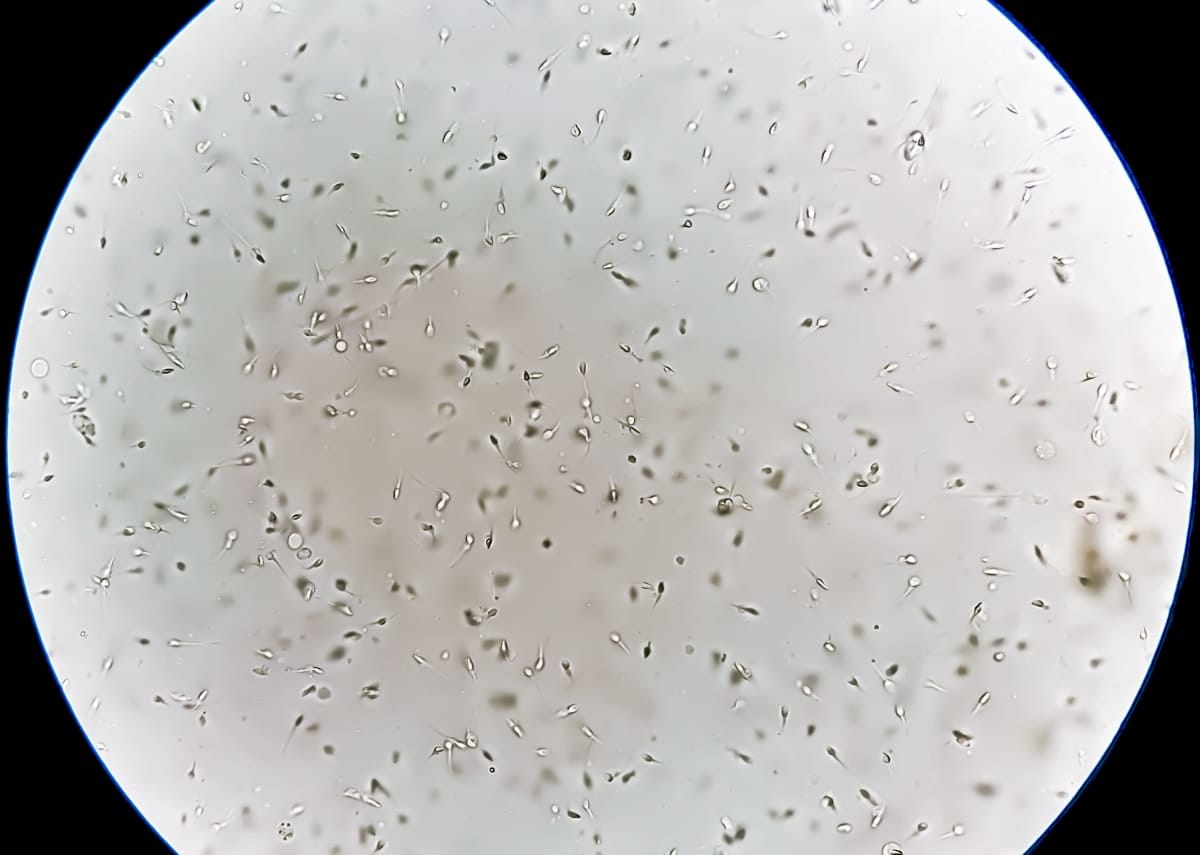
How does sperm behave as a microfluid?
They’re flagellated motile cells – basically a cell that has some kind of a hair-type structure, or cilia, that push them to swim.
If you put them in a very confined environment, because of the hydrodynamic interaction between the flow field that forms near that moving filament and the wall, they start to accumulate and swim next to the wall.
This is important, because the conventional clinical methods being used to select sperm for assisted reproduction now – it's like you put sperm in a big field like, let's say, a soccer field, and you just wait for them to find a way out and leave that environment, and then the ones that leave, they’re the good cells.
What we’re doing instead is directing them a little bit better. Instead of putting them in such a big space, we put them in a small corridor with lots of doors, and as soon as they find a door, they're directed to get out.
Then we give them a higher chance to find the doors, because they’re in a more confined environment and because we direct them more clearly. They need less time to get through that maze.
In order to keep populating the planet?
If you look at many of the developed countries – Australia, Canada, United States, Central Europe – most of these places, each country has a set live birth rate that they need to maintain their population.
If they fall below that, there are two dangers. The population will decline over time, but also the population will get older without enough younger people to take care of the older population.
And then that’s the key motivation, I think, that not only researchers, but governments and policymakers need to look into – pathways to better-support the translation of these technologies to help with a better fertilisation rate.