Firstly, as citizens – but also as clinicians, researchers and medical technology innovators – we’re very concerned with the state of diagnosing COVID-19 in Australia.
The recent outbreaks of the Delta variant in New South Wales and Victoria have seen the discussion on COVID-19 testing shift to include consideration of rapid antigen testing.
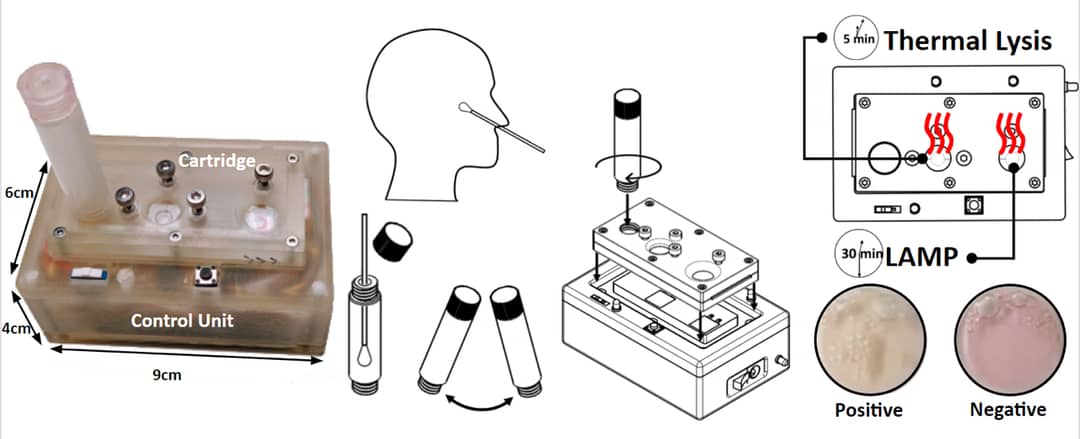
But this only heightens our concern, and further reveals a failure of public policy to invest in development and deployment of decentralised diagnostic devices, most importantly a highly-mobile and swift point-of-care (POC) COVID-19 nucleic acid test.
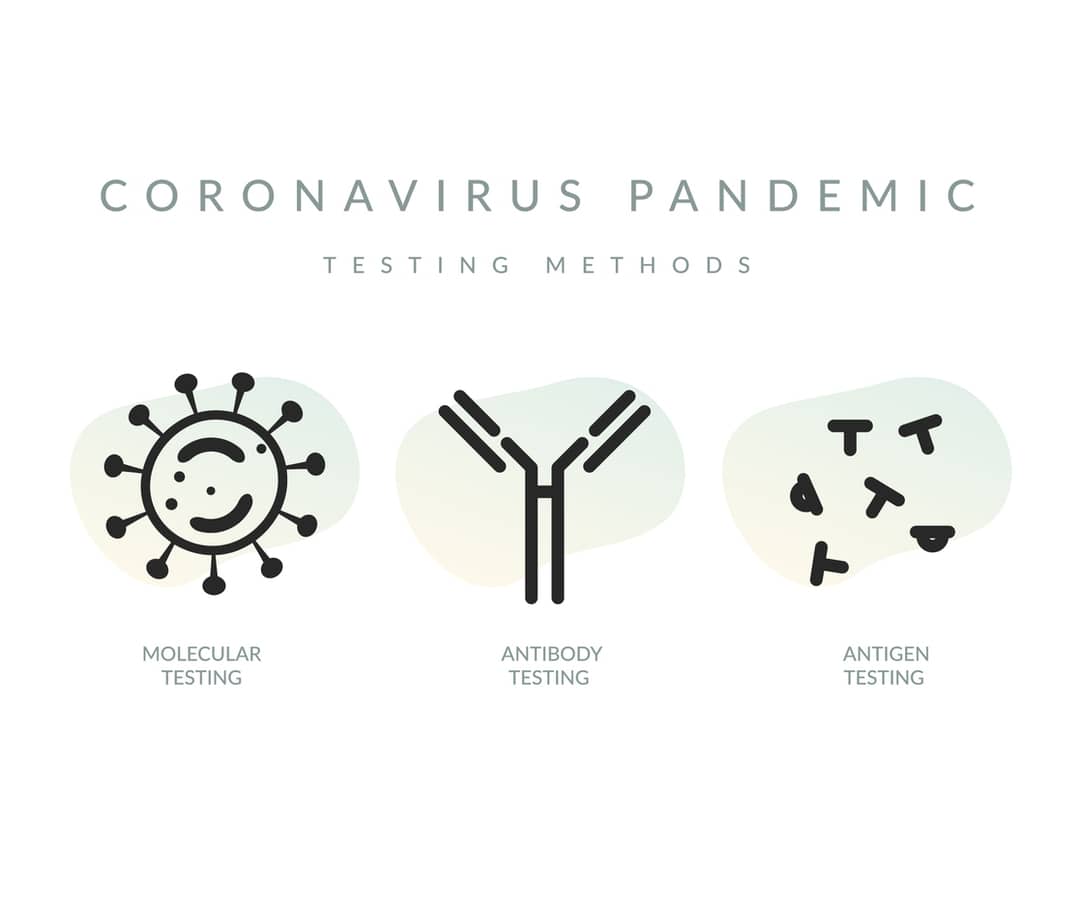
These quick and portable POC tests probe viral RNA in the human body rather than antigen proteins or antibodies, and are a viable alternative to the current gold-standard but expensive, time-consuming RT-PCR tests – the throat and nose swabs most tested Australians have so far undergone.
But we (government, industry, academia, philanthropists and the general public) need to invest in new technologies and new ways of thinking before we see the benefits of them in our communities.
Testing is the first line of defence against COVID-19. However, the current centralised testing model has been exposed as deficient. Anyone who has had testing in the community (as opposed to in a quarantine facility) will know the inconvenience, including long queues (more than 10 hours in the recent NSW outbreak), and the anxious wait for results, during which we need to self-isolate and may not be able to work or provide care to others.
It’s particularly challenging for those with chronic illnesses, disability, and the elderly to endure the travel and wait times associated with the existing centralised testing.
Additionally, people outside urban centres often need to travel long distances to testing sites, and suburban drive-in centres have become notorious for causing traffic gridlock.

Moreover, the delay in receiving positive results (up to four days in large outbreaks) exposes the risk of further transmission, especially during poor compliance with quarantine requirements. This also means the public health response doesn’t have up-to-date information, hampering this response and increasing the risk for further transmission
The size of the problem becomes apparent when looking at figures from the recent Delta outbreak. Between 1 June and 3 August this year, there were approximately 3.3 million COVID-19 tests conducted in NSW, and 1.8 million in Victoria.
The true economic cost for conducting these tests is hard to determine, with a large workforce and much infrastructure required to manage a centralised sample collection program.
Beyond the actual fee for the sample processing, which some estimates have at between $25 and $100 per test, there are costs associated with venue hire, traffic management and security, sample collectors, transportation of samples to the accredited pathology laboratories, and notification of results to the individuals tested and their healthcare providers.
Beyond also the “direct” cost of centralised testing, there’s lost productivity, and, of course, the significant cost of social impacts.
Recent unpublished modelling work from our group shows that in Victoria and NSW there are 6,116,456 workers, of whom 3,792,203 (62%) work full-time. Productivity Adjusted Life Years (PALYs) gauge productivity losses (workforce dropouts, presenteeism and absenteeism) associated with ill health that accumulate over time in a population, leading to significant societal and economic consequences.
If we assume a conservative estimate that one month’s worth of productivity has so far been lost due to the pandemic, the cost in Australia can be estimated as equivalent to $55.8 billion lost in GDP.
Read more: PALY ally: Calculating the cost-effectiveness of healthcare
We argue it’s likely that point-of-care (POC) testing can reduce some of this productivity loss.
We also know from recent modelling work that POC tests – compared with laboratory-based tests – showed a high net monetary benefit, especially if accuracy is high and the wait time for results short.
'Benefits of a POC test may result from fast turnaround times and greater accessibility, with the potential sites for testing expanding to include, for example, pharmacies, GP offices, nursing homes, rural and mobile health clinics and workplaces.'
These gains accrue through, for example, reduced staff time and improved productivity by minimising time off work while getting a test and isolating while waiting for results. The latter can be particularly frustrating for the majority of individuals who are low-risk and asymptomatic.
Like vaccine production, it’s in Australia's national interest to develop its own truly point-of-care COVID-19 nucleic acid (not antibody or antigen) test, rather than relying on imports from other countries.
Read more: Shifting focus on Australia’s COVID-19 vaccination rollout
Many existing POC tests require large desktop equipment to be operated by highly-skilled personnel for sample processing or readout, and would be more appropriately referred to as “near POC”.
A true POC test should be completely portable and can be used by untrained individuals following simple instructions. The increasing interest in POC medical diagnostics has attracted significant attention to self-contained, portable devices, which can diagnose diseases outside hospital settings.
POC testing is “performed near or at the site of a patient, with the result leading to possible change in the care of the patient” or patient management.
Most importantly, the sample delivery, processing and detection capabilities should be integrated in a single unit, and provide a “sample-in, answer-out”-type response with a high precision and a rapid turnaround time under an hour.
Benefits of a POC test may result from fast turnaround times and greater accessibility, with the potential sites for testing expanding to include, for example, pharmacies, GP offices, nursing homes, rural and mobile health clinics and workplaces.
A true POC test requires little or no training in their use, enabling the expansion of testing into the community. This may allow resources to be reallocated in support of remote and vulnerable populations – for example, those in low socio-economic environments who are disproportionately affected by the pandemic, in part due to poor access to centralised care.
Deficits in mobility can impact an individual’s capacity, and willingness, to attend centralised testing sites. POC testing can redress some of these concerns among cohorts such as those living in regional and rural areas, the elderly, and those with certain disabilities.
This is important for those of us concerned with distributional justice – that is, how the harms and benefits accruing during the pandemic are distributed.
A nucleic acid POC test could also help improve health and wellbeing across society, and, importantly, for the most vulnerable members of the community, including improved access to healthcare goods and services.
A POC test may also help address the significant impact the pandemic is having on those workers precariously (casually) employed, who cannot work remotely, or who provide essential services.
'To avoid us getting in the “back of the queue”, it’s of strategic importance that Australia develops its own POC tests.'
In some instances, these factors accumulate – for example, in the aged and disability care sector, which has a highly casualised workforce providing essential care to those at high risk of COVID-19 infection. Access to a workplace POC test could radically improve safety, overcome testing hesitancy, and reduce distress for carers and those for whom they care.
Workplace testing also has other obvious applications, such as for construction sites and industries that require onsite labour, allowing mandatory testing to occur rapidly with minimal impact on productivity.
This is in stark contrast to the recent NSW experience where workers often waited and isolated for several days after a COVID-19 test. Testing at “borders”, such as between states, LGAs and workplaces, could also reinstate freedom of some movements.
It could be argued that Australia can simply import POC tests developed in other countries. However, as witnessed in the under-supply of vaccines, relying on imports places us at significant risk of failure to meet surges in demand.
To avoid us getting in the “back of the queue”, it’s of strategic importance that Australia develops its own POC tests. Even though numerous antibodies or antigen-based tests are demonstrated to provide rapid detection at remote settings, they cannot match the sensitivity offered by the current gold-standard nucleic acid amplification tests (NAAT).
The standard antibody-based tests are useful only once a detectable amount of antibodies are produced in the peripheral blood of the infected person. Similarly, antigen-based tests, monitoring specific proteins from the infectious virus, are much less sensitive than the gold-standard RT-PCR-positive samples from COVID-19 patients.
The ideal device for Australia should also be able to detect multiple pathogens (and their mutations) after simple modifications. This currently isn’t possible with antibody or antigen tests.
Development of these devices will require significant investment, but we think the amount is minimal when compared with the economic loss and social impact of recurrent lockdowns.
So, why isn’t one available yet?
We don’t know, but it may be due to valid concern with accuracy for the antigen and antibody tests. There may also be an assumption that “high throughput” laboratory testing is always more cost-effective, without considering the infrastructure needed, and the loss of productivity due to waiting both to be tested and for the return of results.
We’ve shown these concerns are misplaced.
More variants of COVID-19 could be on the way. There’s potential for ongoing transmission despite effective vaccines. Therefore, high volumes of testing will be required for the foreseeable future.
While there’s been much welcome investment in developing vaccines, there’s been minimal federal funding to develop better testing technologies, despite testing being the first line of defence against COVID-19.
We calculate that the development of POC nucleic acid COVID-19 testing, including an initial development plan, feasibility study, concept demonstration and automation, is expected to cost less than $5 million, which is equivalent to the economic loss associated with lockdown during the time it takes to read this article.
According to KPMG, the lockdowns in Sydney and Melbourne are costing the economy more than $2 billion a week. To reduce the economic and social impact of the pandemic, we call for greater investment in developing POC nucleic acid testing methods that are comparable to PCR in accuracy.
Currently, Australia has all the relevant infrastructure, industrial, and research expertise to achieve this goal.
All we require now is the political will.





