
More than two million years ago, Australopithecus africanus lived in South Africa during a period of major climatic and ecological change. This human ancestor had both human and ape-like traits, and sits in the family tree just before the origins of our own genus, Homo.
While the first australopithecine fossils were found almost a century ago, an international research team, including Monash’s Dr Luca Fiorenza, Dr Justin W. Adams and Associate Professor Alistair Evans, have been able to unlock the secrets of how they raised their young. In a new study published today in Nature, the parenting habits of one of our earliest extinct ancestors are revealed for the first time.

“This is an amazing window into the world of our ancient ancestors, revealing the life and times of an infant millions of years after it died,” said Dr Evans, an expert in the evolution of teeth at the Monash School of Biological Sciences.
The team used specialised laser techniques to vaporise and analyse microscopic portions on the tooth surface.
Teeth are covered by enamel, the most mineralised tissue in our body, which makes them extremely hard and particularly resistant to destruction and decomposition. This is why teeth are the most abundant remains found in archaeological and fossil sites. They're the only elements of our skeleton that come into direct contact with the environment, and so they provide precious information about diet, health and cultural habits of extinct species and past human populations.
During our growth and development, trace elements from the food we eat, the water we drink and the air we breathe are incorporated into the mineral structure of teeth; and once tooth enamel forms, it doesn't change.
Anthropologists and palaeontologists can take advantage of this, because teeth form by adding layer after layer of enamel and hard tissue every day. Thus, teeth are particularly valuable for reconstructing the events occurring during the early life of an individual, simply because they preserve precise changes and chemical records.
Human and other primates are generally characterised by a slow life – they reach sexual maturity at relatively late ages, they live long lives and, at the same time, have a low fertility rate.
Read more about South African Neogene and Quaternary palaeontology
Great apes – orangutans, gorillas and chimpanzees – invest a considerable amount of time and energy in parental care, and breastfeed their babies for a long time. Orangutans, in particular, nurse each baby for more than eight years before weaning, with a gradual replacement of breast milk by other foods. Prolonged breastfeeding leads to long intervals between births, making great apes extremely vulnerable to extinction.
On the other hand, modern humans – which are also very slow to mature – wean their infants at an early age (around 2.5 years in pre-industrial societies), and therefore they can reproduce at a much faster rate than great apes.
This makes weaning an important part of how quickly the next baby can be born. Understanding the weaning patterns of a species can provide accurate information about the length of time that an animal relies on its mother, and about the rate at which reproduction is occurring.
"The chemical signatures locked in the teeth show us what young fossil humans were eating from day to day, month to month."
In the new study, two-million-year-old teeth from Australopithecus africanus fossils were analysed by Dr Joannes-Boyau at the Geoarchaeology and Archaeometry Research Group at Southern Cross University in New South Wales.
The research team then analysed the stable isotopes (chemical elements) of important elements in these teeth, which do not radioactively decay over time.
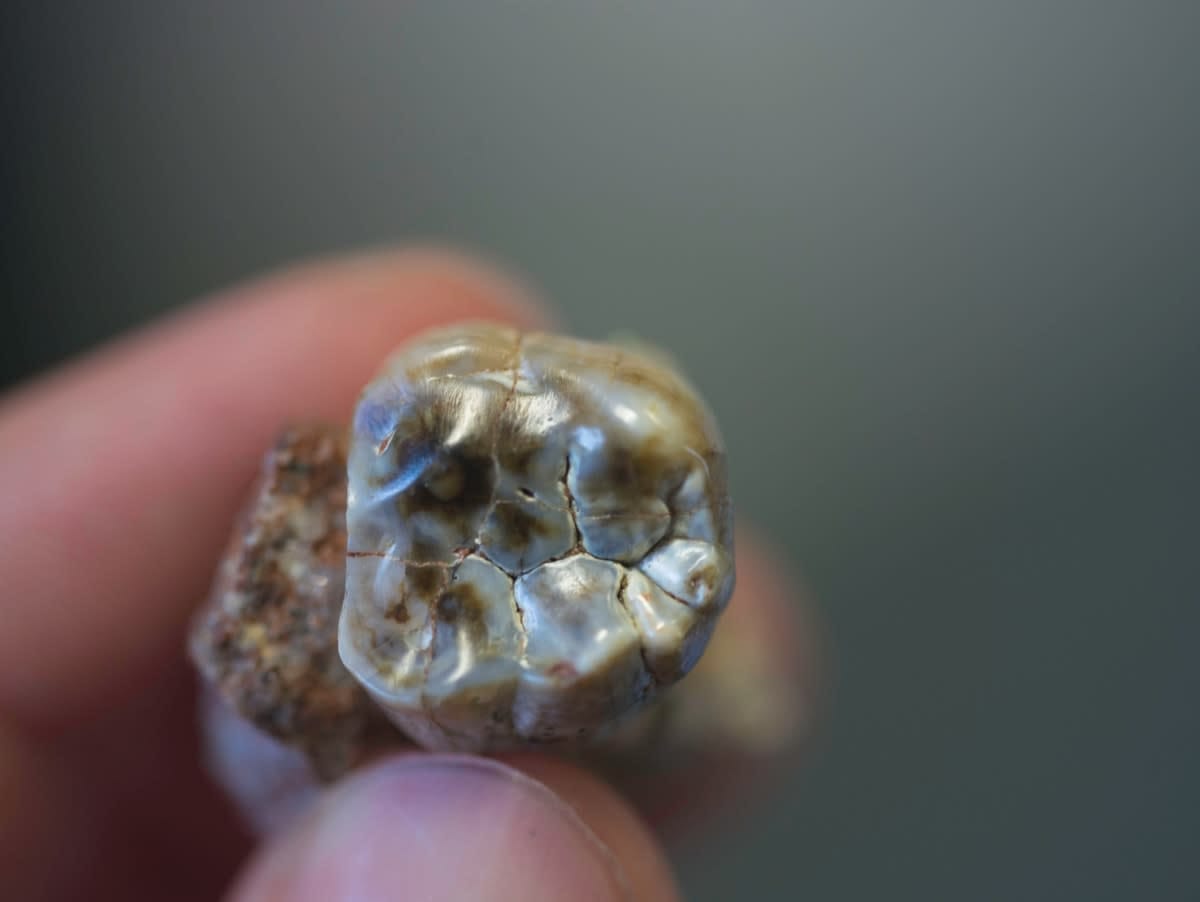
The analysis of stable isotopes is based on the principle that "we are what we eat", so the isotopic composition of food eaten is recorded in the body tissues. The sample is analysed for chemical signatures with a mass spectrometer, enabling researchers to develop microscopic geochemical maps to tell the story of the diet and health of an individual over time.
The research team looked at the distribution of specific trace elements, such as barium, to reconstruct the nursing behaviour of our extinct ancestors. A previous study has shown that the levels of barium in teeth correspond with increases in the mother's milk intake, and then slowly decreases during weaning, reaching its lowest level when the infant’s diet is based solely on solid foods. Because barium is chemically similar to calcium, it's rapidly absorbed and transported into the bloodstream from milk, and ultimately incorporated into enamel and dentine during mineralisation of the infant’s teeth.
Their results have shown that barium, lithium and strontium elements increase for the first year after birth, and then they follow a cyclical pattern, suggesting that infants of Australopithecus africanus were breastfed continuously for the first 12 months of their life. But instead of fully weaning their infants, nursing appears to have continued in a cyclical pattern in the early years for infants.
Adaptive strategy
The use of breastfeeding as a supplemental food source for their growing infants represents a unique adaptive strategy in our evolutionary lineage, and would have allowed for greater infant survival on a landscape with seasonal food shortages.
“These findings suggest for the first time the existence of a long-lasting mother-infant bond in Australopithecus. This leads us to rethink the social organisations among our earliest ancestors,” said Dr Fiorenza, who's an expert in the evolution of human diet at the Monash Biomedicine Discovery Institute (BDI).
Dr Adams, an expert in hominin palaeoecology and South Africa sites at the Monash BDI, said the discovery that Australopithecus africanus mothers were relied upon to provide nutritional supplementation for their offspring and use of fallback resources highlighted the survival challenges of early human ancestors in the past environments of South Africa.
“The chemical signatures locked in the teeth show us what young fossil humans were eating from day to day, month to month,” he said.





