Everyone has a unique identity and awareness of ‘self’ – and this identity has a biological counterpart at the cellular level. This cellular ‘awareness’ is mediated by genes in the human leukocyte antigen (HLA) family, and these genes are the reason, for example, that transplanted organs are rejected unless efforts are made to match HLA types between donor and host.
HLA genes are the ones that vary the most between individuals, and they tag the surface of every cell in our body in a way that denotes precisely who and what we are as biologically individual.
There is, however, another side to these molecules, and it’s been an aspect that has baffled immunologists for decades.
Monash University researcher and Monash Health clinician Professor Richard Kitching explains that, normally, the immune system is trained to not attack ‘self’. Occasionally, this immune ‘tolerance’ breaks down and the result is a painful, life-changing or life-threatening autoimmune disease in which the immune system attacks and damages healthy tissue.
What has baffled immunologists, he says, is that certain HLA gene variants dramatically increase the risk of developing a particular autoimmune disease, while other HLA variants provide impressive levels of protection.
Now, Professor Kitching can explain the HLA effect on disease susceptibility with respect to Goodpasture disease – which was used as the research model – in which the patient’s immune system impairs kidney and lung function.
Even more importantly, the experimental systems developed during this research now provide an opportunity to develop better, cell-based therapies to protect against immune kidney diseases. The findings can also be used to test whether the new cell-based approach is applicable to other autoimmune diseases, including type 1 diabetes, rheumatoid arthritis, Crohn’s disease and multiple sclerosis, he says.
Our cellular identity crisis
HLAs are fascinating molecules. Besides denoting ‘self’, they also provide a structure on the cell’s surface to display to the immune system fragments of potentially dangerous proteins, such as material derived from viruses, bacteria or cancers.
Immune cells peruse the material presented by HLAs using specialised receptors. When the receptor can dock to the HLA-presented material, that cell can become active and launch an immune response against anything that resembles the displayed material. As such, HLAs and immune cells work together to survey the body for potential health problems.
Also, different classes of HLAs exist for suspect materials that originate from inside versus outside our body’s cells. Class I HLAs are expressed by all the cells in our body and deal with potentially dangerous material sourced from inside these cells – for example, as a result of cancer. These HLAs interact with specialised immune cells (cytotoxic T cells) that can kill cancerous or virus-infected cells.
In contrast, Class II HLA molecules deal with material that originates outside our cells, such as debris from a bacterial infection. These HLAs are generally expressed only on specialised scavenging cells (called dendritic cells) and interact with a different subset of immune cells. These cells, known as T helper cells, are responsible for the direction of the immune response, including instructing the immune system to produce protective or damaging antibodies.
The seminal research subject, Goodpasture disease, results from the HLA-mediated contact between T helper cells and a fragment of collagen proteins sourced from specialised ‘basement membranes’ (a tissue that provides a scaffolding structure to the kidneys and lungs). This contact results in the production of antibodies that then attack healthy basement membranes. Left untreated, the disease causes kidney failure.
There is, however, an enigmatic twist to this story.
Professor Kitching explains that Goodpasture disease is very rarely found in people whose cells use one of the variants in the Class II family of HLA genes known as ‘DR1’. In dramatic contrast, the disease risk increases by as much as 16-fold in people whose cells rely instead on the DR15 variant.
Until recently, there was no known mechanism to account for these HLA-mediated differences in disease risk.
An enigma solved
Working in the Monash Centre for Inflammatory Diseases, Professor Kitching and his senior research fellow, Dr Joshua Ooi, teamed up with Professor Jamie Rossjohn and his colleagues Dr Hugh Reid and Dr Jan Petersen at the Monash Biomedicine Discovery Institute.
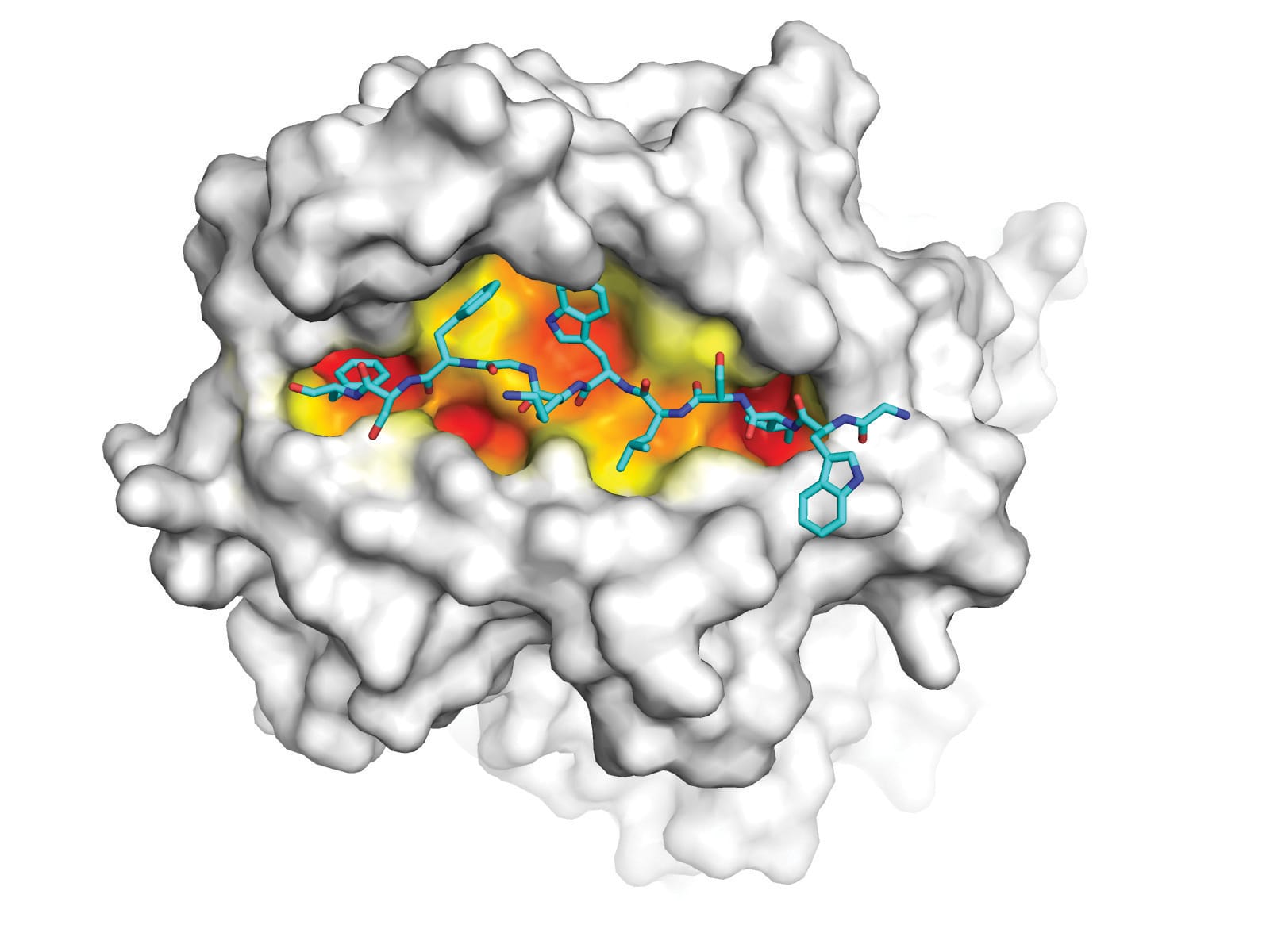
Together they used molecular imaging techniques to compare the structure of the HLA molecules encoded by DR1 and DR15. They discovered that 13 differences in the sequence of these HLA molecules change the way the disease-inducing collagen fragments binds to DR15 and to DR1.
“There are changes to the shape of groove, and the way the collagen fragment fits into the groove,” Professor Kitching says. “That means the collagen fragment looks different from T cells depending on whether it is in the DR1 or DR15 configuration.”
In an experimental tour de force, the two differently shaped HLA-collagen molecules were used as bait to trap, identify and compare the T cells that dock to the DR1 and DR15 configurations.
“We found that the same collagen fragment can activate different immune responses,” Professor Kitching says of the research recently published in Nature magazine. “In the DR15 configuration, the interaction is with T helper cells and can result in self-harming autoantibody production. In the DR1 configuration, however, the immune cell activated is called a regulatory T cell.”
Regulatory T cells are unusual. Unlike most other T cells that are programmed to attack disease threats, regulatory T cells do the opposite – they suppress the immune response.
It is because of this that regulatory T cells are particularly good at dampening the risk of autoimmunity, Professor Kitching says.
“I believe cell therapy based on the new capability to select for specific regulatory T cells is the way to proceed to help patients, and it is something we can pursue here at Monash.”
At Monash Medical Centre, a new building houses the Translational Research Facility, with one floor dedicated to the development of cell therapies. The centre also has a clinical trial unit and two floors of laboratories; another floor houses technology platforms, such as DNA sequencers and cell-sorting flow cytometers.
“Monash now has a powerful stake in a resource that will allow for the research and development of new therapeutics to Good Manufacturing Practice (GMP) standards,” Professor Kitching says. “We have worked up the means to detect and isolate autoimmunity-suppressing regulatory T cells, and we have the option to test them for their ability to suppress and protect against autoimmune-mediated diseases.”





