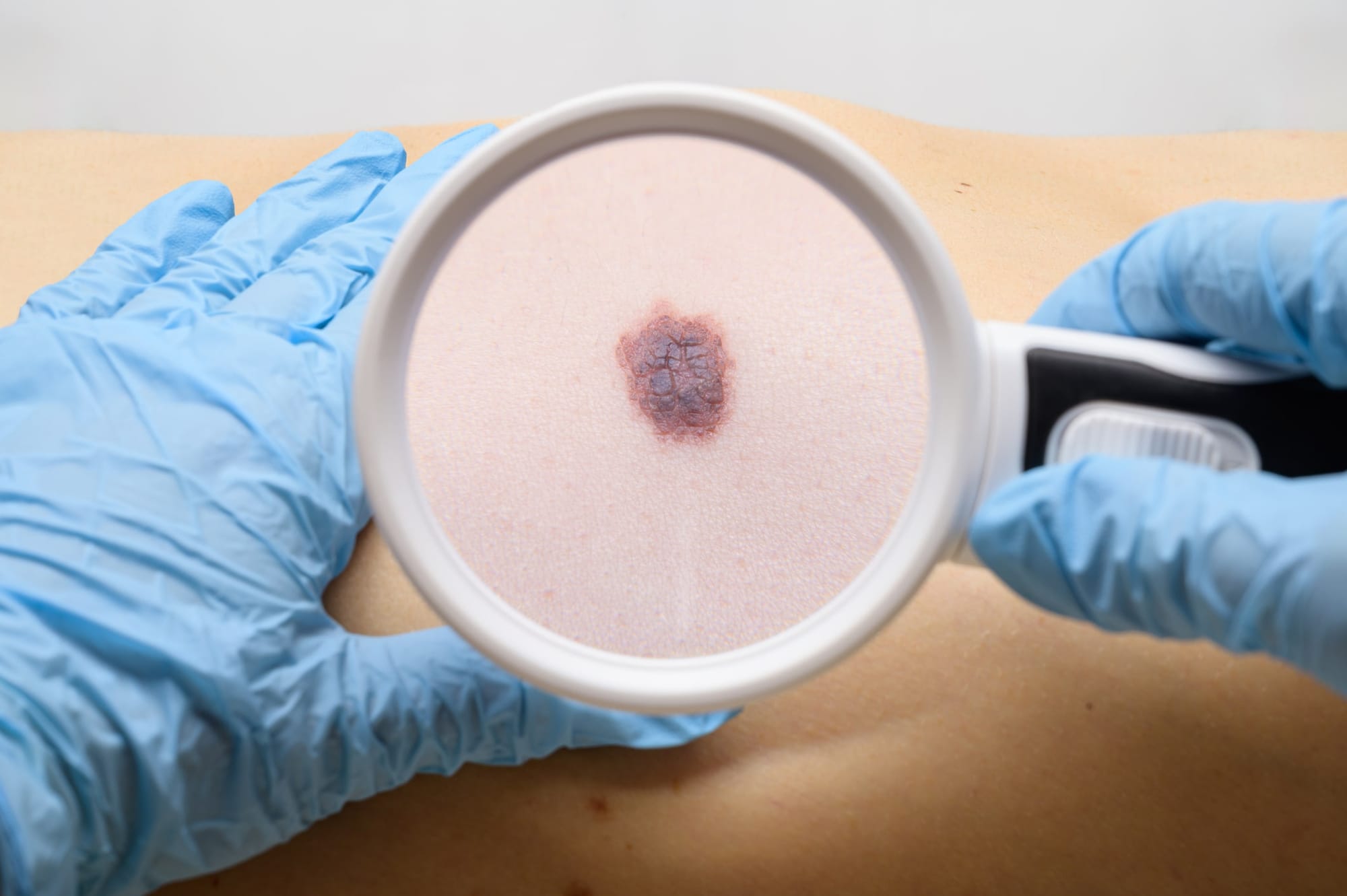
Every year, more than 18,000 Australians are diagnosed with invasive melanoma – the nation’s so-called “national cancer”. Another 28,000 are diagnosed with in-situ disease.
In the early stages, these cancers have excellent recovery rates; the five-year survival rate for stage one melanoma is more than 98%. But thicker, later-stage tumours are more aggressive, require more complex surgery and costly medical therapies, so early detection is a crucial defence.
For decades, well-meaning public awareness campaigns have encouraged people to “get a skin check,” but the reality on the ground is far more complex. Who will benefit from a skin check? Who do people go to – a GP, a skin cancer clinic, a dermatologist, a mole mapping service? How do they know when to seek expert opinion? How do we ensure the accuracy of diagnosis?
And crucially, how can we best serve the population given the critical workforce shortage of both dermatologists and GPs?
These are not trivial questions. They go to the heart of why Australia is leading the way in researching new approaches, such as 3D total body imaging supported by artificial intelligence, to improve melanoma detection and understand risk for a more personalised approach.
But as new data from early studies emerges, so too does a temptation to draw premature conclusions. In doing so, we risk overlooking the long-term promise of innovation if it’s properly evaluated and thoughtfully deployed.
The promise of emerging technologies
That promise is perhaps most evident for rural and regional Australians, who face limited specialist services and long travel distances. For them, the combination of AI, medical imaging, and telehealth holds transformative potential.
These technologies can bring expert-level assessment closer to home and reduce wait times, thereby enabling earlier intervention for people who might otherwise delay care. As AI matures, it may further support local clinicians in triaging lesions and identifying cases that need escalation to specialist review.
Even when patients are seen in-person, diagnostic accuracy can vary widely, at both the clinical and pathological level. In some settings, up to 30 benign lesions are removed for every melanoma detected. And among borderline lesions, even pathologists can disagree about whether a lesion is malignant.
As a result, many people undergo unnecessary procedures, potentially creating pain and harm, and substantial financial burden. At the other end of the spectrum, some face misdiagnosis, and all the personal consequences that entails.
This is not a failure of individual doctors, but of an overstretched system. And it is precisely here that technology can help – not by replacing clinicians, but by augmenting their ability to assess, track, and make informed decisions.
What’s being investigated
High-quality, standardised imaging enables clinicians to detect changes over time – one of the key indicators of malignancy. When integrated with AI tools, such imaging has the potential to reduce variability, identify at-risk patients more precisely, and support more targeted care.
This is the long-term vision behind ACEMID (Australian Centre of Excellence in Melanoma Imaging and Diagnosis), a multi-centre effort to collect rich imaging data across Australia.
The aim is to build a national dataset that reflects our unique population and melanoma risk profile, and then to use that dataset to train and validate artificial intelligence that can support clinician decision-making.
Read more: AI in healthcare education: Is it ready to teach the future?
It’s crucial to understand that this work is being done in stages. We’re currently in phase one – data collection. Images of the whole skin, individual lesions and their corresponding histopathology, genomics and additional metadata are being collected. The AI is not yet in use for clinical decisions.
Melanoma diagnoses in this phase are being made by doctors, not machines. In phase two, we’ll test the AI, and only then will we begin to assess its impact on clinical outcomes.
Let me be clear – the role and value of these technologies in routine clinical care is debated among the dermatology community. ACEMID exists as a platform to understand disease biology as much as to help answer those burning questions about efficacy and cost-efficiency of new diagnostics.
Which brings me back to my warning about drawing premature conclusions.
A long-term research investment
A recently-published, small single-centre study has been cited by some as evidence that the 3D imaging is no better than a standard skin check. But this interpretation misses key context. That study tested a teledermatology model of care – not AI-assisted diagnosis – and aimed to evaluate whether adding remote specialist review could improve access for regional patients.
Unsurprisingly, more lesions were excised in the intervention group.
This is likely due to several reasons. Chief among them is that more professionals assess the patient’s skin. Up to three doctors reviewed intervention group participants, but just one doctor was used in the control group.
Another is a well-known phenomenon called the Hawthorne Effect, whereby the doctor’s decisions may have been influenced by the knowledge they were being studied.
Furthermore, the study was not designed to test the ability of the machine itself to make the diagnosis or flag lesions of concern, and did not use artificial intelligence.
However, international research has tested the machines’ diagnostic capabilities. A Swiss study comparing the diagnostic performance of 3D total body imaging, 2D AI tools, and dermatologist assessment found the 3D device was superior to 2D AI and comparable in accuracy to dermatologists.
This is evidence of a tool with serious promise, not failure.
The AI tools currently being developed with data collected through ACEMID will be fit-for-purpose for the Australian population, and can be integrated into the 3D machines, validated and improved prospectively.

A more standardised approach is needed
Finally, the current narrative regarding overdiagnosis should not obscure the broader goal. Yes, we must avoid unnecessary excisions. But we must also ensure those at real risk of life-threatening melanoma are found and treated early. To do that, we need better risk stratification, more consistent diagnosis, and broader system-level change – a more organised, standardised approach that does not currently exist.
That’s why the ACEMID study, and others like it, are so important. They’re long-term investments in infrastructure, evidence, and innovation. And they’re doing the hard, slow, necessary work of building a better future for skin cancer care in Australia.
No single study will provide all the answers. But if we’re to balance benefit, harm, cost and access for skin cancer detection, we need to stay focused on the bigger picture.
We need to think critically, plan carefully, and keep patients at the centre of every decision we make.





