Each year, a million newborn babies die from severe bloodstream infections known as neonatal sepsis. The most common cause of these infections are bacteria called Klebsiella pneumoniae, and the problem is greatest in low and middle-income countries where infections can be extremely difficult to treat due to high rates of antimicrobial (antibiotic) resistance.
K. pneumoniae are a major player in the global antimicrobial-resistance crisis, and the World Health Organisation’s No.1 priority for enhanced surveillance and novel control strategies.
Not only do these bacteria cause neonatal sepsis, but they’re a major cause of pneumonia, blood and urinary tract infections in elderly and immunocompromised adult hospital patients.
In 2019, antimicrobial-resistant K. pneumoniae were estimated to be associated with 790,000 deaths, but the true burden is difficult to measure, particularly in low-resource settings.
How can we tackle this superbug?
We need better and more connected surveillance to identify and track antimicrobial-resistant K. pneumoniae, so we can understand how it spreads and design effective containment strategies. We also need new ways to treat and prevent K. pneumoniae infections, particularly among the most vulnerable patients.
Vaccinations are an appealing prevention strategy. There are two proposed use-cases – vaccination of expectant mothers to stimulate production of antibodies that can cross the placenta to protect the baby after birth, and vaccination of adult populations at high risk of hospitalisation and subsequent infection.
Read more: Data modelling breakthrough in the fight against antimicrobial resistance
Priority vaccine targets include the K. pneumoniae capsule – the sugary coat on the outside of K. pneumoniae cells that can be recognised by our immune systems. Almost 80 different capsule types have been defined by laboratory techniques, but DNA sequence analyses suggest there might be twice as many.
Each capsule type is recognised differently by the immune system, and this poses a problem for vaccine design, because a maximum of 10-20 capsule types could likely be included. Minimal or no vaccine protection is likely for capsule types that are not included in a vaccine.
But the right combination of capsule types, protecting against at least 70% of K. pneumoniae-causing disease, could prevent 400,000 neonatal sepsis cases and 80,000 neonatal deaths each year.
So how can we know the right combination of capsule types?
Genomic surveillance can help
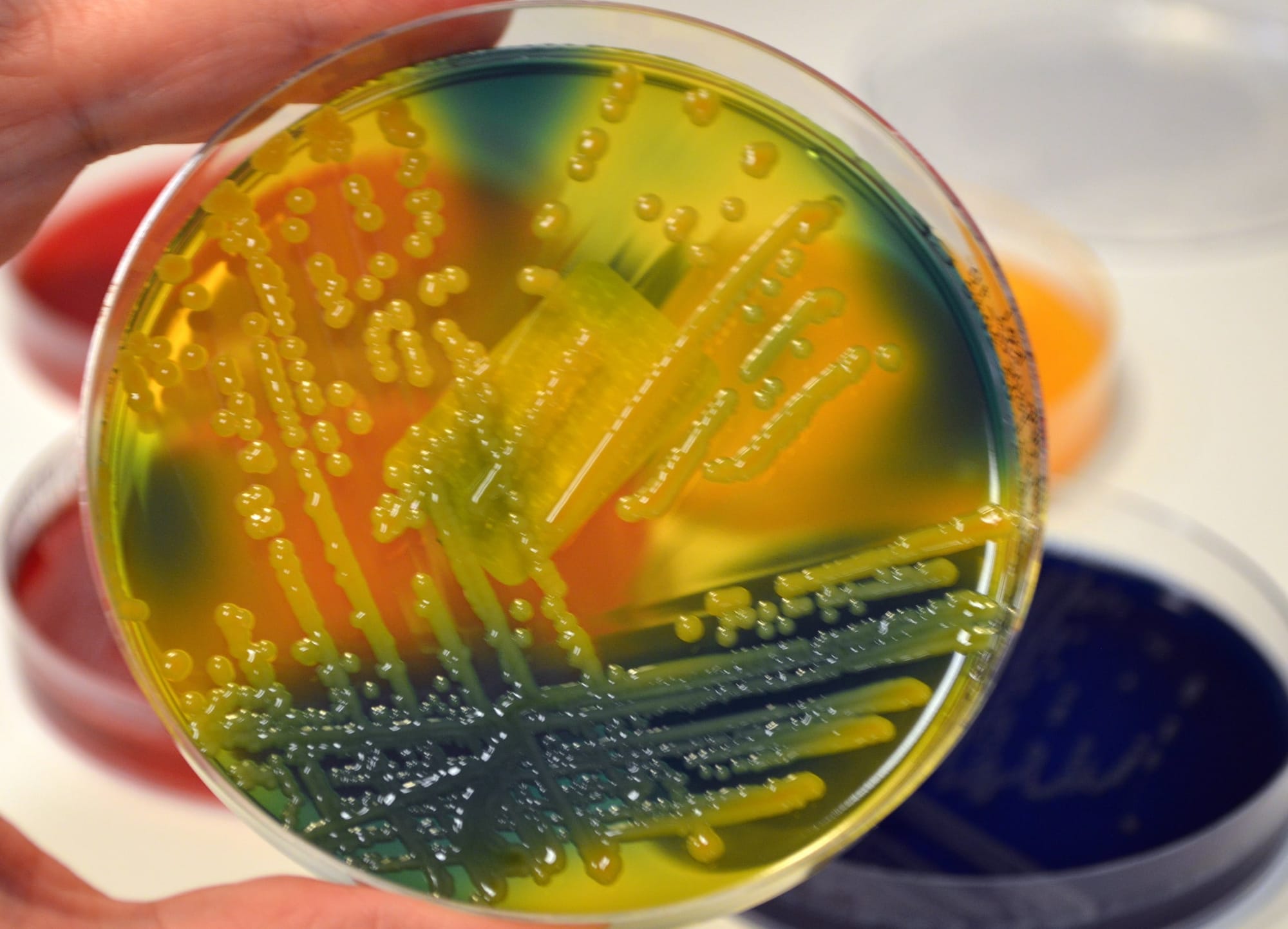
An organism’s entire DNA sequence is called its genome. K. pneumoniae genomes typically comprise 5.5 million DNA bases, from which we can infer a whole suite of information about the individuals they represent.
For example, we can detect genes that make K. pneumoniae resistant to antimicrobials or those that allow them to cause more severe infections. We can also detect and track particular high-risk antimicrobial-resistant “clones” (a similar concept to the widely-publicised COVID-19 variants of concern).
Most relevant for vaccine design, we can detect the genes that encode the K. pneumoniae capsule using a dedicated computational tool developed by our team. Crucially, this allows us to rapidly predict capsule types for thousands of K. pneumoniae, a scale that would not be feasible using laboratory techniques.
Read more: Antimicrobial resistance: Is phage therapy the key to unlocking the superbug crisis?
Together with collaborators at the London School of Hygiene and Tropical Medicine, we’ve applied this tool to predict high capsule diversity among K. pneumoniae-causing hospital infections, and show that antimicrobial-resistant clones can switch capsule types as they evolve.
These findings emphasise the need for broad and ongoing surveillance to inform the design of an effective vaccine, and detect any changes in response to vaccination programs.
Supporting genomic surveillance at scale
Our team are members of the KlebNET Genomic Surveillance Project, a multinational effort to harmonise, improve and expand the accessibility of genomic surveillance tools for K. pneumoniae.
This initiative brings together researchers from Monash University, London School of Hygiene and Tropical Medicine, and five other international research organisations, who recently received US$4.9 million funding from the Bill and Melinda Gates Foundation.
With this funding we’ll expand and improve our existing computational tools, and develop resources that support global genomic surveillance efforts for K. pneumoniae and other bacteria causing neonatal sepsis.
We’ll work with the global K. pneumoniae research and public health communities to develop data standards, and provide educational resources for genomic analyses.
Our Monash University team will develop new resources for tracking high-risk antimicrobial-resistant clones, and build the next generation of genomic capsule prediction tools.
Together with our partners and collaborating teams across Africa and Asia, we’ll apply our tools to K. pneumoniae genomes from neonatal sepsis infections, and create a priority list of capsules for inclusion in a maternal vaccine.





