It was a case of perfect timing.
Scientists had been researching how to use RNA for medical purposes for more than 40 years when the COVID-19 pandemic hit in 2020.
They’d already had some success with an RNA therapeutic approved in 2018 to treat people with the rare hereditary disease familial amyloid polyneuropathy.
But the use of RNA in Pfizer-BioNTech and Moderna’s widely-used COVID vaccines put the molecule on a much bigger stage.
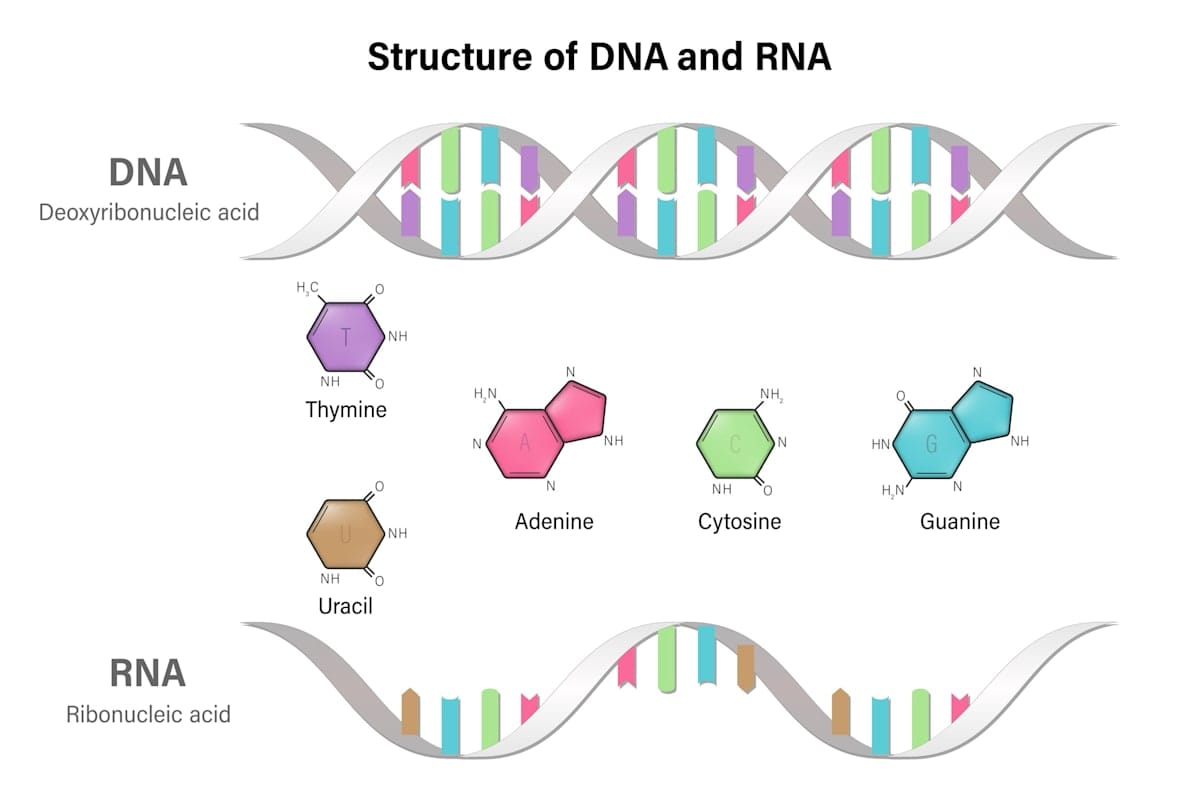
RNA stands for ribonucleic acid. Think of it as the spinoff of the more well-known DNA, or deoxyribonucleic acid.
All humans have naturally-occurring RNA in their cells – the molecule is vital to the everyday upkeep of healthy cells, and acts as the cell’s internal messaging system.
RNA is a set of instructions given by DNA, which is like the boss in the cell's headquarters – the nucleus. These RNA instructions are sent out to the factory floor of the cell – the cytoplasm – for the cellular machinery to follow.
The field of RNA-based therapeutics lets scientists write their own set of instructions for cells to follow.
The different types of RNA allows for different types of therapeutics to be developed.
Arguably, the type of RNA that people are most familiar with is messenger RNA, abbreviated to mRNA.
mRNA is a type of coding RNA. Think of it like an instruction manual that teaches a cell to build a specific protein, similar to what you would get to build flat-pack furniture like a desk or a bookcase.
mRNA therapeutics include the COVID vaccines from Moderna and Pfizer-BioNTech, which give cells a blueprint to make their own copy of the SARS-CoV-2 spike protein in order to train people’s immune systems to recognise and defend against the virus.
Why mRNA vaccines have been so lauded is that they’re often cheaper and faster to make than traditional vaccines, and are generally safer and more efficient at producing an immune response.
mRNA vaccines are not limited to battling infectious diseases
Basically, a vaccine is anything that trains the immune system to fight something.
In cancer, mRNA vaccines provide instructions to make proteins that teach the immune system how to find and destroy cancer cells without harming the healthy cells around them.
MSK mRNA Pancreatic Cancer Vaccine Trial Shows Promising Results https://t.co/NZ9SxRg3KA— Society for RNA Therapeutics (@RNA_Therapeutic) May 30, 2023
These vaccines can be both prophylactic (given to healthy people to prevent certain cancers) or therapeutic (given to cancer patients to assist the body in fighting certain cancers). This is because unlike with other diseases, cancer patients sometimes can’t mount a sufficient immune response on their own.
Despite the complexity of cancer, there are multiple mRNA-based vaccines currently in human trials.
This includes Moderna’s mRNA vaccine against melanoma – currently in phase three clinical trials for use in combination with Merck’s approved cancer drug, Keytruda – which teaches the immune system to selectively take down tumour cells.
Moderna and Merck announced that when messenger RNA (mRNA) technology is combined with cancer immunotherapy drug pembrolizumab, it creates a vaccine that has been shown effective against melanoma. https://t.co/M2y5VU6zSP pic.twitter.com/tWDsY9Hjbz— Melanoma Research (@MelanomaReAlli) January 11, 2023
BioNTech, in collaboration with Genentech, has an mRNA vaccine in phase two human trials to treat colorectal cancer. And the University of Florida is undertaking a phase one clinical trial for an mRNA vaccine to tackle the most common type of brain tumour.
If even one of these vaccines is successful, it will mark the beginning of a new generation of cancer treatments that will hopefully improve patient survival rates.
Beyond vaccines, mRNA can have as many functions as can be written into their biological coding.
One application mRNA is being developed for is protein replacement therapy.
Sometimes cells may naturally lack the capacity to make their own mRNA for a specific protein in-house or the instructions they have are faulty, leading to non-functional or toxic forms of the protein mistakenly being built.
This is the case for certain genetic disorders such as cystic fibrosis, where cells produce a faulty copy of a protein called cystic fibrosis transmembrane conductance regulator, or CFTR, which causes unhealthy levels of mucus to build up in the lungs.
By providing regular deliveries of the correct mRNA to the cells, they can start making the normal protein instead.
RCT2100, a cystic fibrosis mRNA drug developed by ReCode Therapeutics, is one of multiple mRNA drugs currently in human trials for this application.
ReCode Therapeutics nets $80M to push RNA treatment for cystic fibrosis https://t.co/035sUv31hn #ReCodeTherapeutics #RNATreatment #CycticFibrosis #LungDisease #VentureCapital— Haybury (@Hayburysearch) March 28, 2020
Here, an inhaled form of mRNA is introduced to the lung cells to help them produce healthy CFTR proteins, with the aim of reducing the build-up of thick mucus and alleviating the symptoms experienced by cystic fibrosis patients.
If successful, such mRNA therapeutics can address unmet clinical needs and the lack of effective treatments, particularly for people diagnosed with rare genetic disorders.
Another type of RNA is silencing or small interfering RNA, abbreviated to siRNA. This is a type of non-coding RNA, which means it can’t instruct a cell to build a protein.
Instead, siRNA is like a gag order, designed to put a halt to the production of faulty or undesirable proteins.
siRNA instructs cellular machinery to seek out and intercept any copies of a specific mRNA out for delivery, and then tear them in half before they can be read by the cell’s manufacturing systems.
This leaves the cell with unfinished instructions that it cannot build from, and so it discards the mRNA.
Patisiran, for example, is an siRNA therapeutic approved in 2018 to treat the rare hereditary disease familial amyloid polyneuropathy, by instructing cells to stop making a faulty, toxic form of the protein transthyretin.
Many challenges overcome
Over the past 30 years, scientists have faced and overcome multiple challenges in the development of RNA therapeutics.
One of the major breakthroughs has been the development of the lipid nanoparticle delivery system – a synthetic “envelope” for the RNA instructions, protecting it from the harsh conditions in the body, and allowing more precise delivery to targeted cells.
As a result, the field of RNA therapeutics has expanded incredibly.
As of late 2023, there are more than 80 mRNA therapeutics in human trials, with at least five new clinically-approved mRNA drugs coming to market, and many more in the registration process.
This isn’t including the myriad therapeutics in preclinical stages being developed by hundreds of companies and academic institutes worldwide, and medicines being developed using other types of RNA.
While there’s still room for improvement, with RNA therapeutics the future looks bright.
Dr Emily Pilkington and Dr Rekha Shandre Mugan are project managers for mRNA Core, a collaborative initiative to foster the development of mRNA therapeutics towards clinical applications. They’ve received funding for their research from the National Health and Medical Research Council (NHMRC), and the Medical Research Future Fund (MRFF).
Originally published under Creative Commons by 360info™.





