Influenza kills up to 650,000 people around the world each year, with 99% of deaths in children under five years of age in developing countries due to influenza-related infections, according to the World Health Organisation.
The current crop of influenza vaccines has limitations in effectively combating the billion cases of seasonal influenza each year, as they provide immunity against only one specific existing strain or mutation.
The propensity of flu viruses to mutate into new strains means vaccines must be continuously monitored and reformulated each year.
But a universal influenza vaccine, using mRNA technology – which was used with success during the COVID pandemic – has the potential to provide broader and longer-lasting immunity against diverse influenza strains.
The technology allows for rapid development and deployment, and offers versatility in targeting multiple regions of the influenza virus.
New or mutated influenza variants are always a threat, particularly those originating from animal sources.
Pandemics such as the Spanish flu of 1918, which killed 50 million people, and recent outbreaks of the avian influenza (“bird flu”) viruses underscore the persistent threat posed by influenza.
Read more: From the Spanish Flu to COVID-19: Tales from the healthcare frontline
It also underscores the urgent need for a universal influenza vaccine capable of safeguarding against all subtypes of the virus.
In recent years, lipid nanoparticle (LNP)-encapsulated nucleoside-modified mRNA (mRNA-LNP) vaccines have emerged as a potent tool in combating influenza and other infectious diseases.
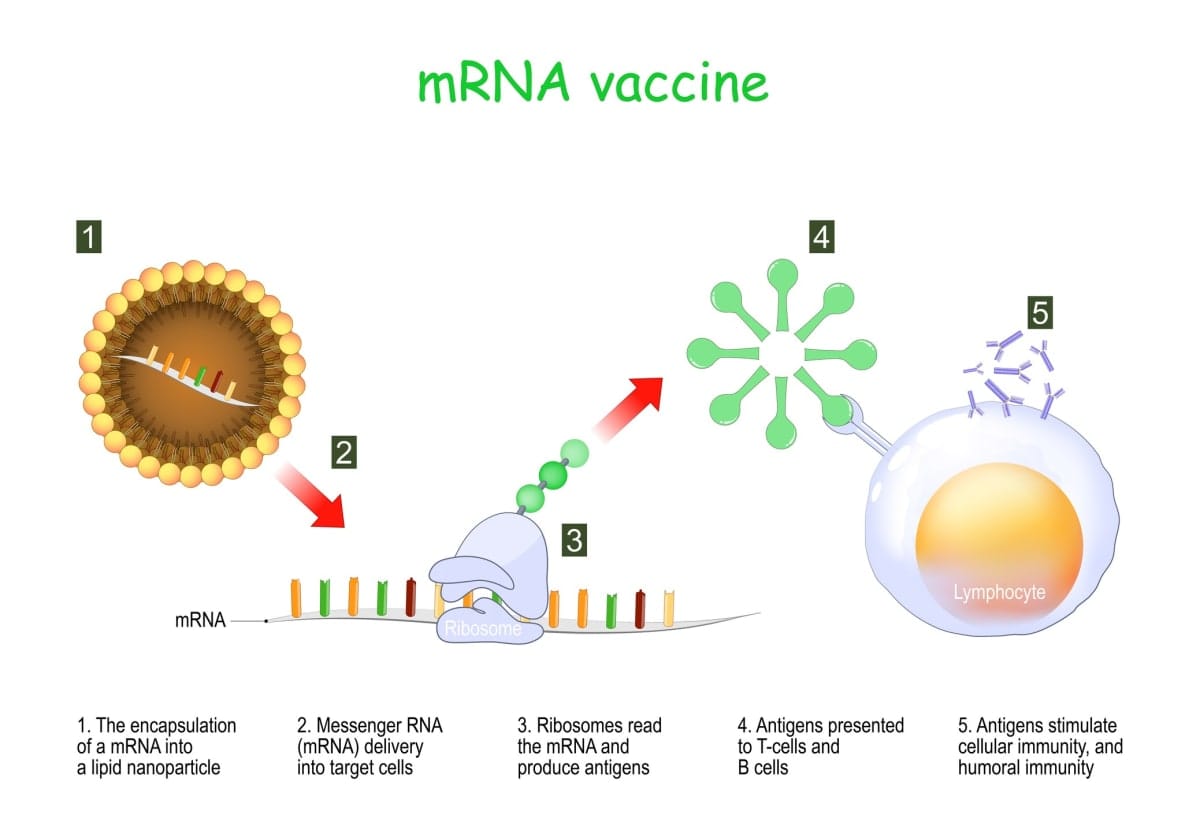
These vaccines use mRNA created in a laboratory to teach our cells how to make a protein – or even just a piece of a protein – that triggers an immune response inside our bodies.
The limited efficacy of current vaccines can be attributed to their focus on only generating strain-specific antibodies against the influenza virus hemagglutinin (HA). This is a protein within the virus that causes infection.
To broaden protective immunity, novel vaccine strategies aim to elicit responses against more proteins (fragments of a virus).
One promising avenue involves triggering T-cell responses. T-cells are a type of white blood cell,and are part of the body’s immune system.
T-cell-mediated immunity not only eliminates infected cells, but also correlates with improved outcomes in individuals affected by influenza. Animal studies have demonstrated the protective role of T-cells against various influenza virus strains.
These vaccines, exemplified by the successful development and global deployment of mRNA-LNP-based COVID-19 vaccines, elicit robust T-cell and antibody responses. These vaccines also offer the advantage of rapid production and adaptation to target emerging viral variants.
While several mRNA-LNP influenza vaccines are in development, most prioritise stimulating antibody responses and under-exploit the potential of T-cell immunity.
How the vaccines can work
The development of a universal influenza mRNA vaccine requires careful consideration of several pivotal factors to optimise its effectiveness and suitability. Initially, the vaccine should target regions within the influenza virus responsible for viral replication, but are less susceptible to mutation.
By concentrating on these regions, the vaccine can elicit broad and enduring immune responses, offering protection against an array of virus strains. Encapsulating nucleoside-modified mRNA within lipid nanoparticles (LNPs) has emerged as a promising strategy to bolster vaccine effectiveness.
These LNPs shield the mRNA from degradation and facilitate its delivery to target cells, where it can be translated into viral proteins, triggering immune responses in the human body.
Additionally, optimising the mRNA sequence can boost protein reaction, ensuring robust and persistent immune responses post-vaccination.
Another crucial aspect of designing a universal influenza mRNA vaccine is incorporating adjuvants or “agents” to amplify vaccine efficacy. An adjuvant is an ingredient used in some vaccines that helps create a stronger immune response in people.
Integrating adjuvants into the vaccine formulation improves vaccine efficacy, especially in individuals with compromised immune systems.
The design of a universal influenza mRNA vaccine should thus prioritise triggering T-cell responses, enhancing mRNA delivery and expression, incorporating adjuvants to boost efficacy, and addressing practical considerations for global distribution.
By tackling these critical aspects, a universal influenza mRNA vaccine holds the potential to revolutionise influenza prevention and control efforts, offering comprehensive and enduring protection against seasonal and pandemic influenza strains.
Confidence in a universal vaccine
As shown by COVID-19, mRNA vaccines have demonstrated safety and efficacy in clinical trials for various infectious diseases. This instils confidence in the feasibility of developing a universal influenza mRNA vaccine that is safe and effective.
Ongoing advancements in mRNA technology, such as improved delivery systems and stabilisation methods, further enhance the prospects of creating a universal influenza vaccine that ticks all the boxes.
The US National Institute of Allergy and Infectious Diseases has started enrolling volunteers at Duke University in North Carolina to test its experimental mRNA-LNP vaccine against seasonal influenza, one of several universal influenza vaccine candidates now in the pipeline. Another clinical trial has begun at the US National Institutes of Health’s Clinical Centre in Maryland.
The mRNA vaccine technology offers several advantages, including rapid development, scalability, and precise design. Two categories of mRNA vaccines exist, conventional and self-amplifying, and both are being explored for their potential to confer broad protection against influenza viruses.
Despite their promise, challenges remain in effectively delivering mRNA molecules into a vaccine due to their inherent instability. Overcoming these challenges is essential for the successful development of universal mRNA vaccines for influenza.
This aside, the advancements in mRNA vaccine technology have paved the way for innovative approaches to crystallising universal influenza vaccination, offering hope for broad protection against this ever-changing virus.
Originally published under Creative Commons by 360info™.





