The immune system is extraordinarily complex and powerful, the primary function of which is to detect and destroy foreign or “non-self” proteins, including infections and cancer.
To do this, it’s evolved two parallel sets of pathways, known as innate and adaptive immunity.
Innate immunity is responsible for fast, powerful inflammatory responses to a wide variety of foreign materials, but has no ability to develop “memory” – that is, to develop very specific and long-lasting recall responses to specific proteins.
The adaptive immune system, in contrast, responds slowly but with huge specificity, resulting in the production of a near-infinite variety of antibodies, including those that recognise a person’s past infections, to swiftly deal with them if they recur.
This is how vaccinations work, and why people need multiple vaccines throughout their lives, because each one is for one specific germ.
It’s a beautiful system, but there’s a problem – the human body itself is made up of thousands upon thousands of "self" proteins that also have the potential to initiate an immune response, leading to “autoimmune disease”.
So why doesn’t everyone have autoimmune disease? And if we can understand that, can we leverage it to help those who do?
A significant immune cell discovery
In 2017, Monash University’s Joshua Ooi, working with Professor Richard Kitching, discovered that people with risk factors for autoimmune disease who don’t develop it are protected by a subset of immune cells called regulatory T cells, or T-regs.
Associate Professor Ooi’s work, published in the prestigious journal Nature, showed that people who are protected from autoimmune disease have a high abundance of antigen-specific T-regs, which powerfully and specifically shut off the immune system’s ability to be activated in response to “self” proteins.
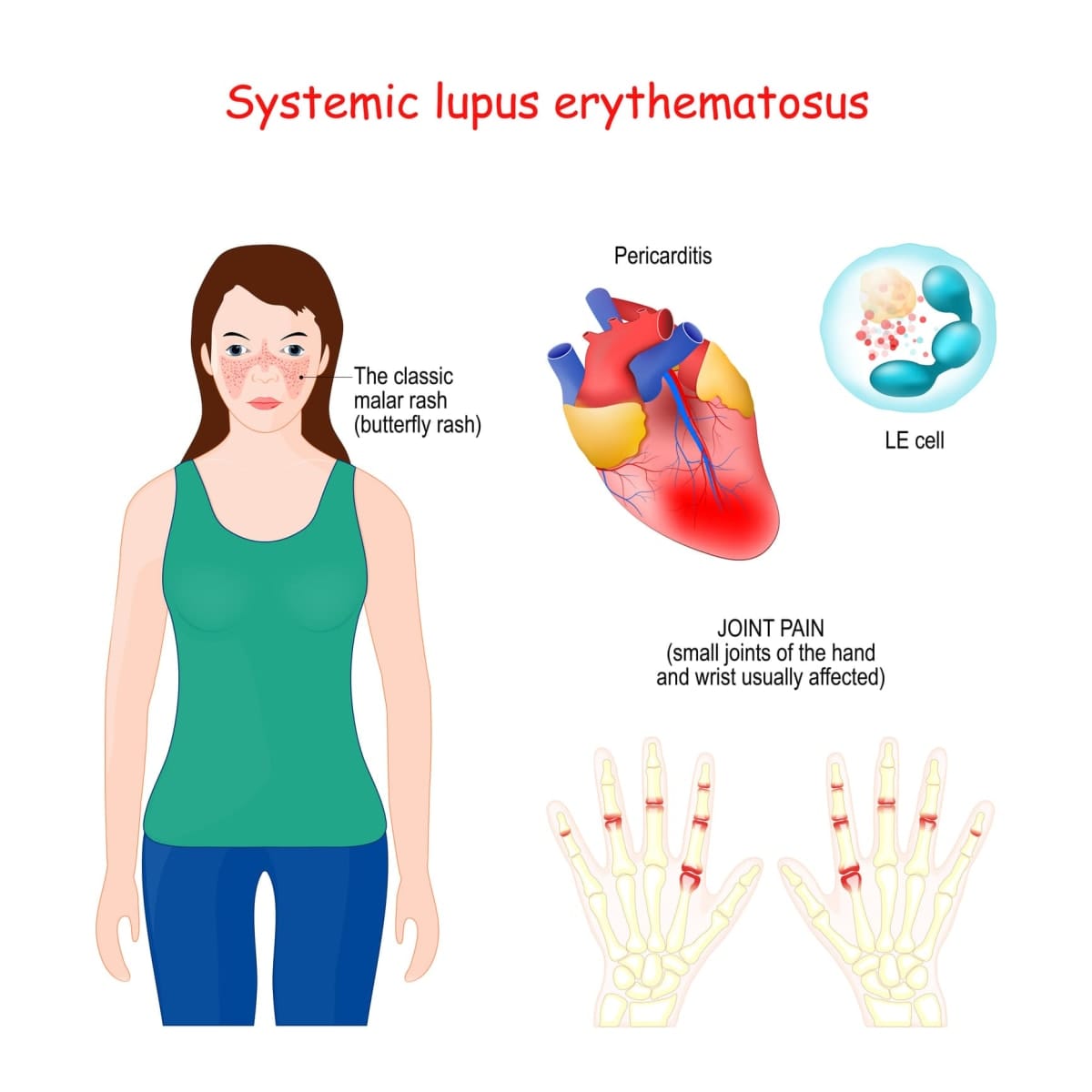
In collaboration with Professor Eric Morand, the team turned to the autoimmune disease systemic lupus erythematosus (SLE, or lupus). Unlike most autoimmune diseases in which a single part of the body is targeted for harm, in lupus, almost any part of the body can be attacked.
Read more: Tackling the rise of autoimmune diseases with genetically modified cell therapies
Lupus is known to be driven by both the innate and adaptive immune system, and indeed the trial that led to registration of the first innate-immune targeting therapy for lupus was led by Professor Morand.
Despite this earlier breakthrough, most patients with lupus are still treated with non-specific immune suppressive drugs and steroids, with poor long-term outcomes and reduced survival – lupus primarily affects young adult women. This is a devastating state of affairs.
Like other T cells, T-regs have a receptor on their surface, the “T cell receptor” or TCR, which is the means through which they identify their target and operate with extremely high specificity.
Protein link to disease outcomes
Professor Morand and Associate Professor Ooi discovered that a large proportion of lupus patients have antibodies to a protein called “Sm”, which is tightly linked to worse disease outcomes. The Sm protein is found in healthy people, too, meaning healthy people must have specific T-regs with an Sm-specific TCR that protect them.
In their new work, published this week in Nature Communications, they’ve achieved the remarkable feat of identifying the Sm-specific TCR in healthy people without lupus, and giving patient T-regs the information they need to express this protective TCR – turning their T-regs into the same powerfully protective T-regs that keep healthy people healthy.
After proving that these “SMART-regs” strongly and selectively repress anti-Sm immune responses in the test tube, Professor Morand and Associate Professor Ooi went further, generating a living model of lupus using immune cells from lupus patients, and showing that the Sm-specific SMART-regs could prevent development of severe lupus kidney disease.
Development for clinical trials
This technology has the potential to rapidly be developed for human clinical trials, with the team planning to be ready to commence by 2026.
In these trials, a patient’s blood would be treated in the lab to produce the protective T-regs and then infused back into them – a so-called autologous cell therapy.
The effects would be expected to suppress only the disease-causing immune response, without the broad immune suppression and accompanying infection risk of current therapies.
Not only that, this technology can be used across any of the approximately 100 autoimmune diseases known to have a similar pathology, meaning it’s a platform for generating autologous cell therapies for millions of patients worldwide.
Read more: Treatment offers new hope for lupus – and maybe for other autoimmune diseases, too
This type of breakthrough can only come from intensive long-term collaborations between clinicians, scientists, and patients.
These collaborations are the raison d’etre of the newly-formed Sub-Faculty of Clinical and Molecular Medicine at Monash University, which represents the University’s partnership with Monash Health and the Hudson Institute, located just 900 metres from Monash University’s Clayton campus, at the Monash Medical Centre.
It’s just one of many translational breakthroughs increasingly recognised by industry and investors coming from the sub-faculty, which has developed a strong entrepreneurial culture in parallel to its focus on human disease.