- The breakthrough lithium-sulfur battery outlasts the lithium-ion battery, and is rechargeable hundreds of times without failing.
- The new generation lithium battery stores two to five times as much energy by weight.
- The new battery uses readily available sulfur and is far more environmentally sustainable and cheaper to produce.
Just imagine for a moment that you could buy an electric vehicle that only needed to be charged once a week. Now imagine that this battery was also clean to produce and made right here in Australia.
Monash University researchers have taken a giant leap towards claiming this holy grail of renewable energy by creating a new lithium-sulfur battery; redesigning the heart of the battery to promote exceptionally fast lithium transfer and improved lifetime performance.
“It’s world-leading,” says Professor Matthew Hill, Deputy Head of the Department of Chemical and Biological Engineering at Monash University.
“A lot of our research has been about making the battery more stable and lasting longer, so this particular discovery is really exciting.”
As the world charges towards cleaner and greener energy, swapping dirty fossil fuels for emissions-free electrification, lithium batteries are playing an increasingly vital role as storage tools to facilitate energy transition.
They’re the go-to choice to power everything from household devices such as mobile phones, laptops and electric vehicles to major industries such as aviation and marine technology, but until now, long-duration storage has been somewhat elusive.
The new frontier of renewable energy
In addition to their environmental benefits, they offer higher energy density and reduced costs compared to the previous generation of lithium-ion batteries, and they can store two to five times as much energy by weight.
Previously, the electrodes in lithium-sulfur batteries deteriorated rapidly and the batteries broke down, but the new interlayer developed by Professor Hill, Dr Mahdokht Shaibani, Professor Mainak Majumder and PhD candidate Ehsan GhasemiEstahbanati from the Faculty of Engineering solves that problem, delivering high capacity and long-life.
“The interlayer stops polysulfides, a chemical that forms inside this type of battery, from moving across the battery; polysulfides interfere with the anode and shorten the battery life,” Professor Hill says.
“It means the battery can be charged and discharged hundreds of times without failing.”
Read more Electric vehicles are on the way, but it's more than a matter of plug and play
While the world has embraced the development of lithium batteries as a game-changer on the path to reducing global emissions, there is a dark side to the clean, green image.
Lithium-ion batteries rely on metals such as cobalt, nickel and manganese, which have finite reserves and are often mined in countries known for poor mining practices and reliance on child labour. The horrific price of green energy has been well-documented.
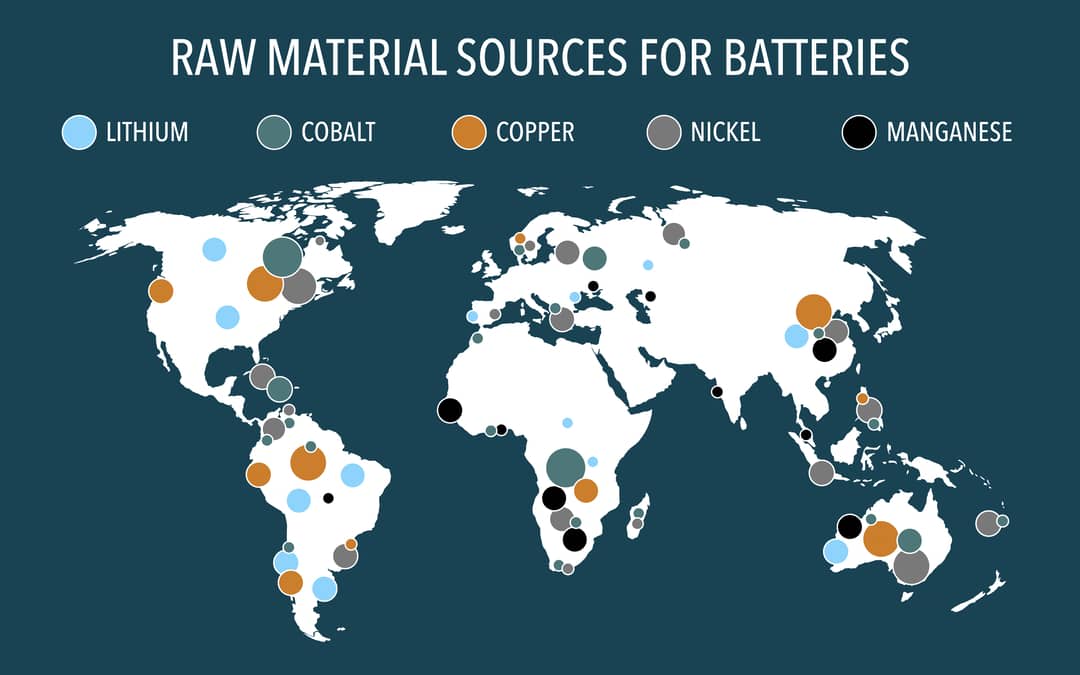
Currently, about 60% of the world’s cobalt supply comes from the Democratic Republic of Congo, where large numbers of mines are unregulated, and the use of child labour is common.
In some cases, children as young as seven have been used to mine unstable tunnels, breathing in cobalt-laden dust.
By contrast, the mineral sulfur is in abundant supply in Australia and almost considered a waste or by-product, and Australian mining practices are among the world’s best.
“We needed to find a better way,” says Professor Hill, “and these batteries are not dependent on minerals that are going to lack supply as the electrification revolution proceeds.
“A good supply of minerals combined with reducing the footprint from mining to manufacturing means this battery is far more environmentally sound and ultimately cheaper to produce.”
The key to this latest discovery was going against the accepted norms and conventions of lithium battery construction.
“It’s ground-breaking technology, because in a regular lithium battery there’s about half a dozen components that make up the battery. Most people research those, but hardly anyone has looked at changing the interlayer or separator in the middle. They tend to be fairly standardised, and until now we’ve assumed it didn’t matter too much if you changed them.
“Now we’re saying it actually does; we’re going against the accepted convention.”
This latest breakthrough, published by the Royal Society of Chemistry, continues the world-leading work into lithium battery development by the Monash team.
World Engineering Day for Sustainable Development is 4 March.