In Australia, heart failure affects 500,000 people with more 50,000 new diagnoses annually. Globally, more than 64 million patients are affected, and with an aging population the prevalence is rising.
Heart failure is typically a progressive condition and patients commonly experience worsening quality of life, frequent hospitalisations and, ultimately, reduced life expectancy.
Existing heart failure interventions
In the advanced stages of heart failure, heart transplantation continues to be the best long-term treatment, yet patient need greatly exceeds the availability.
But there is an alternative solution; Mechanical Circulatory Support (MCS) devices. MCS devices are small mechanical pumps that either assist the native heart in pumping blood, or completely replace the native heart with a blood-pumping device.
MCS devices are increasingly used to treat patients with heart failure, however, problems continue to be experienced by MCS users. These relate to design limitations, including:
- Poor physiological adaptation due to single speed or pulse rate pumps that do not meet the patient’s changing blood flow and oxygenation requirements
- Poor blood compatibility due to mechanical design systems that change and damage blood constituents and expose patients to severe postoperative complications and increased risk of mortality
- Infection due to permanent skin-crossing components used to power devices
- Size limitations - MCS devices are not suited to all forms of heart failure, particularly those in older patients, some women and children, and
- Challenges in using MCS devices where both the left and right sides of the heart are severely damaged.
The daily routine of patients with an MCS device is one of increased cognitive and emotional load, with heightened awareness and relentless anticipation, physical burdens, and practical constraints.
A patient with an MCS device has an external power source and controller connected to the pump by a skin-crossing cable (a driveline), and these peripheral devices must be carried by the patient continuously.
These patients need careful monitoring and perform numerous tasks which are mandatory for continued safe and independent living.
How does the Artificial Heart Frontiers Program help?
The Artificial Heart Frontiers Program (AHFP) brings together world-leading clinicians, engineers and scientists to develop long-term solutions for all patients with heart failure.
Led by Monash University, the program includes University of New South Wales, Griffith University and University of Queensland – as well as The Alfred hospital, the Baker Heart and Diabetes Institute, and St Vincent’s Hospital, Sydney, and major industry partner in BiVACOR.
The AHFP has identified three superior, life-changing devices to address the unmet need of patients with heart failure. These transformative devices will save millions of patients globally, improve their quality of life, increase their productivity, and are anticipated to improve life expectancy.
The BiVACOR Total Artificial Heart (TAH) is a revolutionary, Australian-made, implantable mechanical device, designed to replace the entire function of a failing human heart.
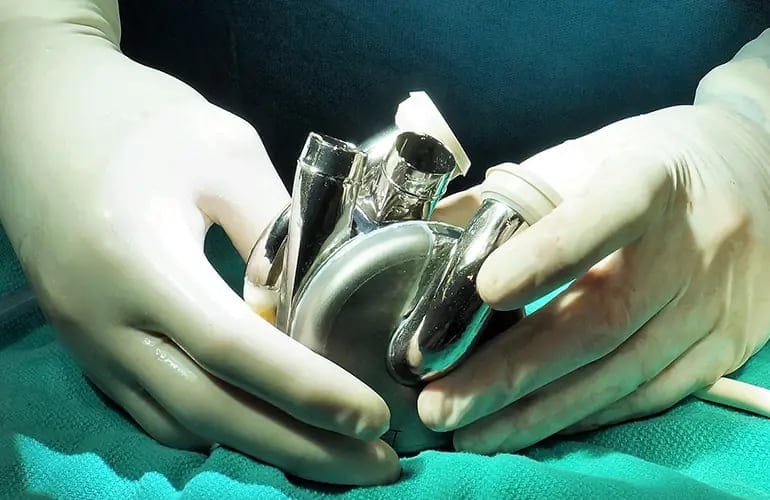
The BiVACOR TAH has world-leading technology, including an optimised hydraulic system to support both sides of the heart; powerful magnetic levitation (MAGLEV) that enhances durability and biocompatibility, with a compact size to support more patients; and automatic flow adaptation that responds to patient requirements without user input.
The BiVACOR TAH provides a platform technology for an advanced Left Ventricular Assist Device (LVAD), a mechanical heart pump that supports the left side of the heart to move blood through the body, supporting the native heart’s remaining capability.
The AHFP is also developing a desperately needed MiniPump, which is a miniaturised pumping device specifically designed to support the native heart of patients with Preserved Ejection Fraction (HFpEF), for which there are no other options.
This is achieved by unloading the left atrium and reducing back pressure on the lungs. The MiniPump would offer a unique treatment option for HFpEF, which now accounts for half of all HF cases – nothing of its kind currently exists.
The technology advances being developed in the AHFP are not limited to the three devices – development has commenced on a pipeline of peripheral complementary products: a wearable controller, infection-resistant driveline, Smart Advisor for clinicians, mobile phone application and website for patients, online feedback portal for clinicians, customisable wearables for patients and enhanced surgical tools and clinical training platforms for surgeons.
Australian healthcare system savings
The AHFP is a game-changing opportunity to deliver solutions for advanced forms of heart failure, as well as deliver substantial health benefits to patients, and economic benefits to Australia.
Development and delivery of AHFP’s novel innovations over a 15-year period from 2022 to 2036 is expected to generate at least $1.8 billion of impact to the Australian economy. This includes savings to the healthcare system, industry expansion in research and manufacturing, creation of jobs and giving Australian patients early access to clinical trials and emerging lifesaving technologies.
Positioning Australia as a global leader
A research grant of $50 million from the Medical Research Future Fund, announced by Federal Health Minister Mark Butler, will not only help to revolutionise implantable heart devices, thereby addressing unmet patient need; it will also position Australia to be the home of next-generation cardiac devices and peripheral systems.
By creating a thriving industry of cardiac innovation and investment, the AHFP will be able to respond to future needs by delivering an expert manufacturing base with a nimble, talented group of workers in this growing industry.
Access to AHFP technologies will save millions of lives globally, and improve quality of life and productivity. This will significantly reduce pressure on the Australian and global healthcare system while retaining and further attracting innovators to create a new Australian ecosystem of cardiac innovation - elevating Australia as a global MedTech leader.