For the first time in decades, people living with schizophrenia will have access to a new class of medicine following the US Food and Drug Administration (FDA) approval of the drug Cobenfy on 26 September.
Cobenfy – originally referred to as KarXT – is a combination therapy of two drugs (xanomeline plus trospium) that works to simultaneously improve symptoms of schizophrenia while also mitigating debilitating side-effects. It represents an entirely new class of more targeted medicines for the treatment of schizophrenia, along with other difficult-to-treat neuropsychiatric and neurological diseases.
First major advancement in decades
Schizophrenia is a poorly-understood and often devastating disease, affecting approximately 1% of the population. On average, people living with schizophrenia die15 to 20 years prematurely due to comorbidities, with the economic annual cost of these comorbid mental and physical health conditions estimated at around A$15 billion.
Much of this can be attributed to a serious lag in resourcing of critical mental healthcare infrastructure. However, until now, all available medicines for the treatment of schizophrenia are:
- based on science that dates back to the 1950s
- essentially have a similar mode of action that predominantly focuses on receptor proteins in the brain for the neurochemical, dopamine
- manage only a subset of symptoms associated with the disease
- are often accompanied by intolerable side-effects.
There has, thus, been an urgent need for a new generation of medicines that are both more effective and safer than those currently available.
How scientists came together to develop the new drug
Developed and driven to market by US-based biotech company Karuna Therapeutics, which was recently acquired by Bristol Myers Squibb, Cobenfy represents an approximately 30-year collaborative journey across industry and academia, including researchers from Monash University’s Faculty of Pharmacy and Pharmaceutical Sciences (FPPS), who have made significant contributions to paving the way for the development of what has ultimately become the first new class of medicine for schizophrenia in more than 30 years.
It all began in the early 1990s, when scientists from pharmaceutical companies Eli Lilly and Company and Novo Nordisk discovered molecules to selectively target M1 and M4 muscarinic receptors, two important members of the superfamily of G protein-coupled receptors (GPCRs – the largest drug target class of proteins encoded by our genome) that recognise the neurotransmitter, acetylcholine, and are well-known to be important in regulating cognition and other neurological effects linked to both Alzheimer's disease and schizophrenia.

However, muscarinic receptors had proven relatively intractable to selective targeting because the primary (orthosteric) binding site for their endogenous agonist, acetylcholine, is highly conserved across all five muscarinic subtypes.
Nonetheless, the Lilly/Novo collaboration led to the discovery of the drug xanomeline, which had enough of a “preference” for M1 and M4 muscarinic receptors to be further developed by Eli Lilly, and progressed into clinical trials, specifically for the treatment of Alzheimer’s disease.
These trials were eventually stopped due to a large side-effect burden, but an interesting observation from those trials was that a subset of the Alzheimer’s patients demonstrated a significant reduction in their symptoms of psychosis – the first clue towards a different picture.
Monash’s research into M1 and M4 receptors
Around the same time, still in the early ’90s, Professor Arthur Christopoulos, FAA, FAHMS, who is now the Dean of Monash’s Faculty of Pharmaceutical Sciences, had commenced his PhD studies on muscarinic receptors in the brain, but looking at alternative modes of targeting them by focusing on secondary “allosteric” sites, which are spatially distinct from the classic “orthosteric” site recognised by the body’s cognate hormones/neurotransmitters.
Allosteric sites offer the potential for achieving greater drug selectivity between related proteins. He extended these studies as a postdoctoral fellow in the US, specifically investigating the molecular pharmacology of xanomeline as one of his first projects.
Because of this research and some of his early discoveries in this space, Professor Christopoulos and his team would often collaborate on a number of projects with key members of the team of scientists behind what has now become Cobenfy.
What followed was 25 years of considerable efforts across multiple academic groups, including key groups in Australia (for example, the Monash Institute of Pharmaceutical Sciences, and the Florey Institute of Neuroscience and Mental Health), as well as industry, to target the M1 and M4 receptors as an entirely new approach to treating schizophrenia and other psychotic disorders, as opposed to the traditional approach of targeting dopamine receptors.
Throughout the 2000s and into the 2010s, the Analytical and Structural Neuropharmacology Laboratory within the Monash Institute of Pharmaceutical Sciences (MIPS), Faculty of Pharmacy and Pharmaceutical Sciences, co-led by Professor Christopoulos and Associate Professors Celine Valant and David Thal, worked in collaboration with Dr Christian Felder and his colleagues at Eli Lilly to continue to validate xanomeline and allosteric modulator drugs as novel treatments for schizophrenia, with a particular focus on targeting the M4 receptor (in addition to the M1 receptor).
It was during this time, in 2009, that some of the lead US scientists left Eli Lilly and kickstarted Karuna Therapeutics, backed by the Boston-based PureTech Health, to focus predominantly on the game-changing potential of xanomeline in schizophrenia.
In 2016, a landmark study led by the MIPS team and published in Nature solved the first high-resolution crystal structures of both the M1 and M4 muscarinic receptors, thus paving the way for structure-based design of selective drugs for treating psychosis, cognitive deficits and related disorders.
Meanwhile, the Karuna team were tackling the unwanted side-effects they’d seen in the initial xanomeline Alzheimer’s clinical trials. They overcame this by pairing xanomeline with another drug, trospium, that works in an opposite fashion to block muscarinic receptors.
The thinking was that trospium could alleviate unwanted side-effects of xanomeline in other parts of the body that contain muscarinic receptors but, because it doesn’t cross from the blood into the brain, would still allow xanomeline’s antipsychotic effects to remain intact. They started clinical trials with this combination therapy, and KarXT (xanomeline-trospium) was born.
As the KarXT trials were progressing and showing promising results, Karuna re-engaged the Monash team in 2018 to help them better-understand the molecular pharmacology of xanomeline.
From 2019 onwards, the Monash researchers and their collaborators revealed why xanomeline is different to other drugs with similar structures, and why it’s more selective for the M4 (in particular) and M1 receptors, relative to the other muscarinic receptors.
Notably, in 2023 – in what was a first for the entire GPCR field – the Monash team solved the atomic-level structure of the M4 receptor bound to xanomeline to show that it interacts with both classic (orthosteric) and secondary (allosteric) binding sites at the same time. Prior to this, the discovery of two molecules of the same drug concomitantly binding to one GPCR had never been seen before, let alone for an FDA-approved GPCR medicine.
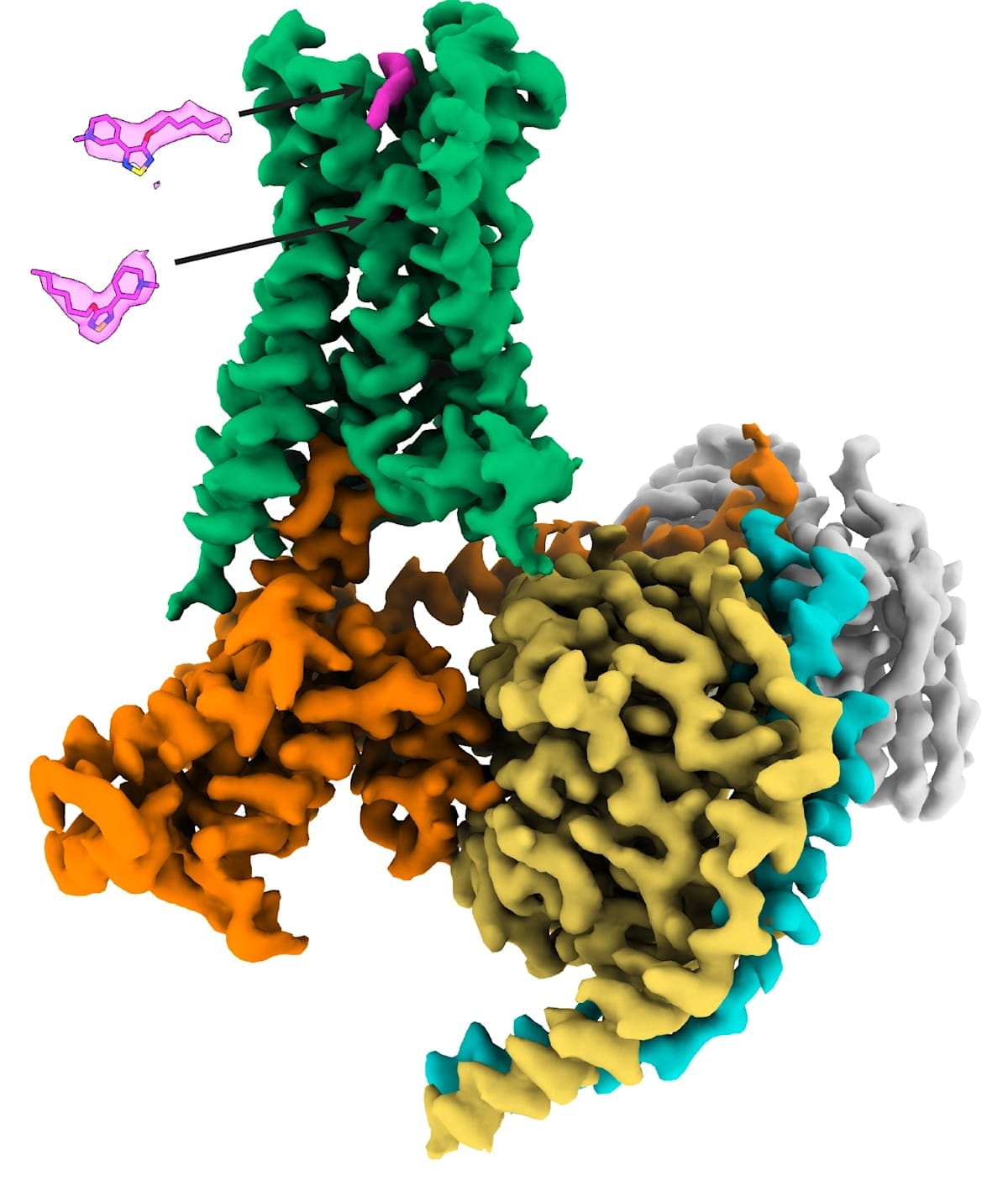
FDA approval of Cobenfy, and what it means for Australia
Professor Christopoulos says that “now, with the approval by the FDA of Cobenfy as the first-in-class antipsychotic medicine of its kind in 30 years, it certainly feels like a case of ‘back to the future’ for me”.
More importantly, however, he states that:
“... this really represents a game-changer for the millions of people living with schizophrenia, as well as for their families and carers, because it opens the door for newer and hopefully safer and more efficacious treatment options.”
But the work is ongoing. At Monash, Professor Christopoulos and his colleagues are continuing to study the molecular mechanisms of xanomeline and related drugs, as well as focusing on how to improve on the therapeutic potential of M1 and M4 muscarinic receptors by developing more targeted allosteric modulators for these proteins, with the ultimate goal of designing even better first-in-class treatments for schizophrenia and other neurological disorders.
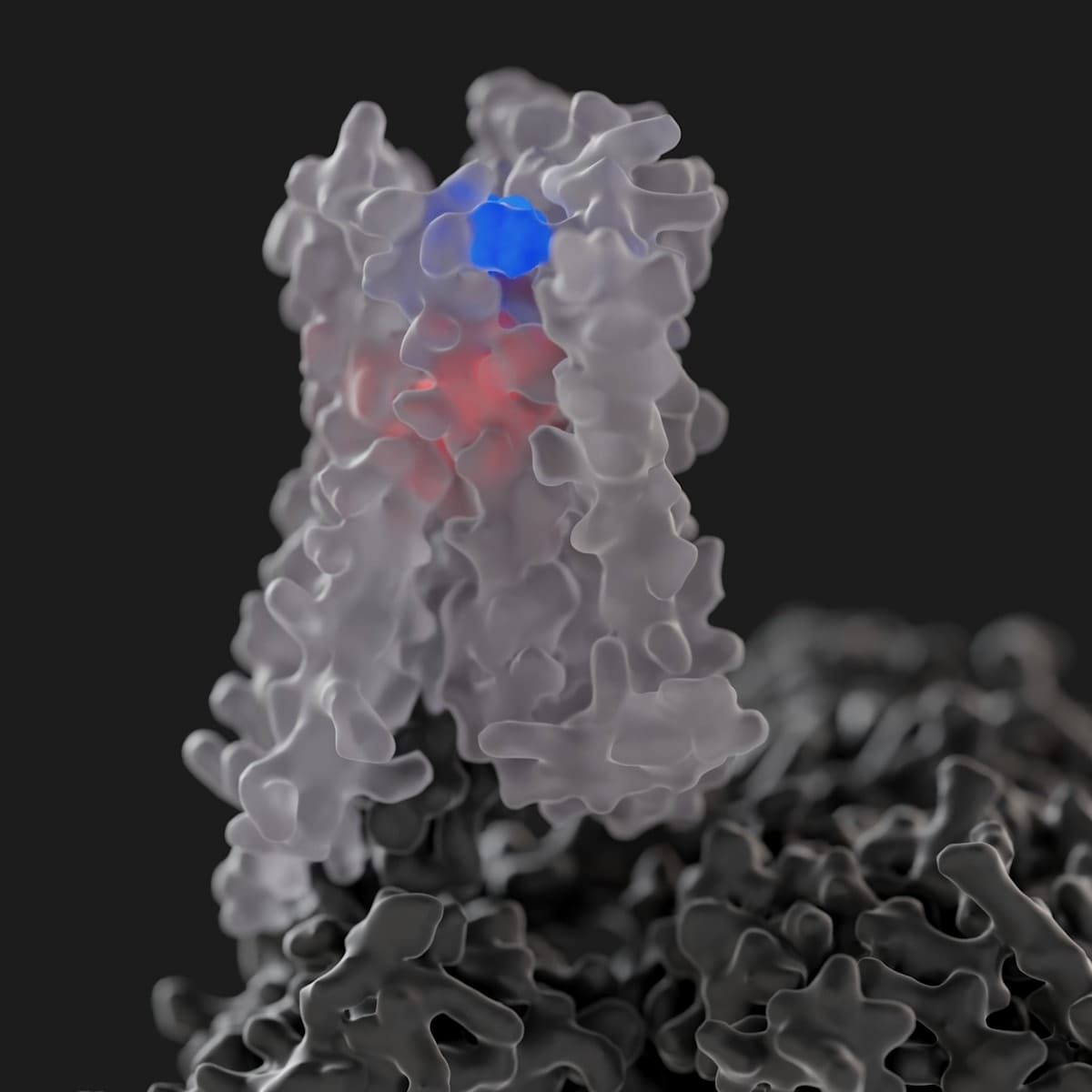
“The Cobenfy story represents exciting clinical validation that we were, and remain, on the right track for developing a whole new class of neuromedicines,” concludes Professor Christopoulos.
“But this is still the beginning in other ways. For instance, Cobenfy still needs to get TGA approval for use in Australia, its price may be a barrier to access without PBS listing, and there will remain a subset of patients for which it cannot be used.
“But, make no mistake, this is an amazing outcome. I wish to congratulate our colleagues and collaborators at Karuna Therapeutics for this incredible breakthrough, and our team at Monash continues to build on this work towards the next generation of even more targeted antipsychotic medications.”