Australia has reached a historic milestone by receiving its first pre-clinical research and training licence in mitochondrial donation, a groundbreaking IVF-based technique that has the potential to reduce the risk of some forms of mitochondrial disease.
The approval from the Embryo Research Licensing Committee (ERLC) follows more than two years of ethical and regulatory preparation to ensure the pre-clinical work begins under strong safety and governance standards.
With this licence, the mitoHOPE Program – led by Monash University in partnership with several research organisations, including The Murdoch Children’s Research Institute and Monash IVF – can now begin training embryologists in the technique using donated eggs.
“For the ethics and licensing committees, this represents two firsts for Australian research,” says Professor John Carroll, leader of the mitoHOPE Program and Director of the Monash Biomedicine Discovery Institute.
“It’s the first time embryos are being created through mitochondrial donation for research, and the first time egg donors are participating solely to support that research.”
How mitochondrial donation works
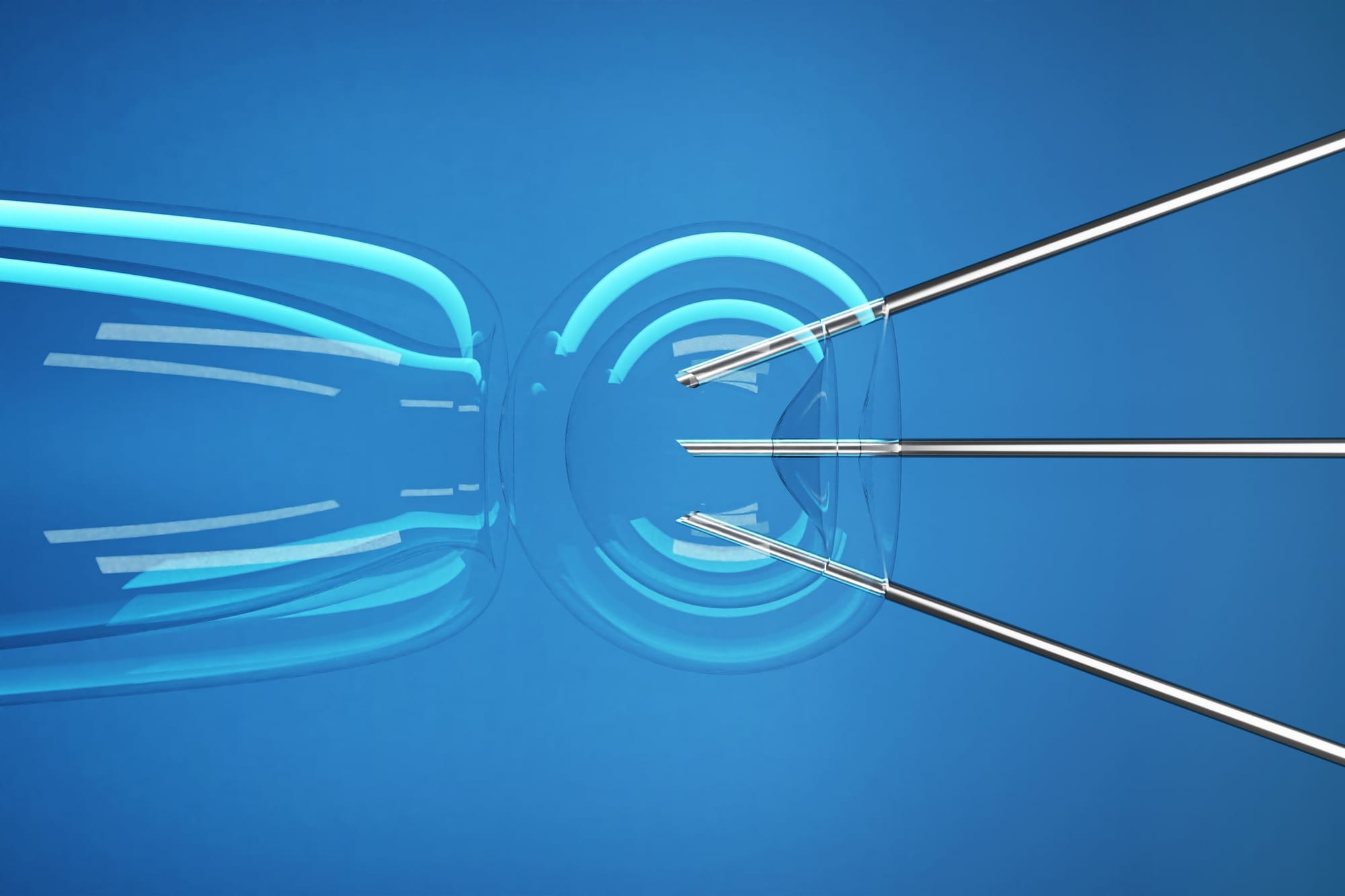
Mitochondrial donation works by replacing disease-causing mitochondrial DNA from a mother’s egg with healthy mitochondrial DNA from a donor.
The resulting embryo carries the mother’s and father’s DNA, but has healthy mitochondria, reducing the risk of passing on mitochondrial disease.
The disease is caused by mutations in the small fragments of DNA contained in the mitochondria. These are transmitted from mother to child, but the risk of disease is unpredictable. Women with no symptoms may unknowingly have it, then pass it onto their children who can suffer premature death, chronic disability or, at best, long-term ill health.
At least one in 250 Australians carry a disease‐causing mitochondrial DNA mutation that puts them at risk of mitochondrial disease during their lifetime.
Australia joins the United Kingdom as the second nation in the world to allow mitochondrial donation for research. The technique is already underway at the Newcastle Fertility Centre in the UK, where eight babies have been born free from signs of mitochondrial disease.
Research coming to fruition
Professor Mary Herbert, a reproductive biologist from Monash's Biomedicine Discovery Institute and the mitoHOPE Program, says it’s been “enormously satisfying” to see two decades of scientific research result in the birth of children in the UK with a greatly reduced risk of developing mitochondrial DNA disease.
Professor Herbert came to Monash in 2023 with a wealth of experience as a professor of reproductive biology at the UK’s Newcastle University and also as scientific director at Newcastle Fertility Centre, where she led the development of mitochondrial donation in the UK.
She’s also a fellow of the UK Academy of Medical Sciences and Royal College of Pathologists.
“I hope that the new treatment can soon become available to Australian families affected by these devastating diseases,” she says, adding “the licence represents a welcome first step towards trialling mitochondrial donation treatment in Australia”.
The long advocacy journey
Bec Walsh, from Toowoomba, living with mitochondrial disease, has been a passionate advocate for getting this pilot program off the ground. She describes her condition to Lens as “a debilitating genetic disorder that robs my body’s cells and organs of energy”.
“Everyone is different, but in my case I struggle with fatigue, muscle pain, shortness of breath and gastrointestinal issues.”
Years of campaigning in Australia by the grassroots “mito” community, including Walsh and advocacy organisation Mito Foundation, led to Maeve's Law in 2022, named for Victorian girl Maeve Hood, who has Leigh syndrome, a mitochondrial disease, with her cells not making enough energy for her to thrive.
“I was heavily involved in advocating for Maeve's Law; I think I spoke to about 50 or 60 different politicians,” says Walsh. “To now see the licence announcement actually happen feels a bit like watching a door crack open that we’ve been knocking on for years. Knowing embryologists can finally move away from mouse egg trials and begin work with human eggs gives families like mine something we haven’t had in a long time – a sense that real change is moving toward us, not away from us,” says Walsh.
“For us, this program isn’t just a scientific step, it’s a lifeline. It means future families may be able to avoid the grief, fear and uncertainty that mito brings.
“And even if this option isn’t something that my family has to leverage personally, the idea that it might one day protect someone else’s child fills me and my husband with a kind of quiet hope we’ve held onto since the day I was diagnosed.
“For our family, mito donation represents far more than scientific progress. It offers genuine hope for some of the most amazing families we’ve come across in an area that is often burdened by fear, uncertainty and loss.”
First step towards donation approvals
This licence is the first of several needed before mitochondrial donation can move into clinical practice.
Once mitoHOPE embryologists have completed their training and the team has met all regulatory requirements, including developing strict safety and consent processes, and approval is granted to begin a clinical trial, participants who meet the eligibility criteria will then need to obtain individual approval from the ERLC to take part.
Mitochondrial donation will only be offered through this tightly regulated research program, with long-term monitoring to ensure safety and ethical standards remain central at every stage.
The Australian government has committed $15 million through the Medical Research Future Fund to support the mitoHOPE pilot program.





