My office becomes less tidy as the days and weeks and months pass – never the opposite. Life is a battle against disorder. This is thanks to the second law of thermodynamics – disorder in the universe tends to increase over time.
Which brings us to my specialty subject - enzymes. There is no life without enzymes. They are the protein molecules that perform all the functions of living things. Enzymes speed up, or catalyse, the chemical reactions necessary for all life on earth, from microbes to humans. The fastest enzyme is so fast that it can reduce the time of a reaction that would normally take a trillion years – a hundred times the age of the universe – down to a hundredth of a second.
They can be linked closely to the idea of disorder, and also order. Enzymes achieve these amazing feats by helping atoms come together in space, working temporarily against the second law of thermodynamics that ensures that eventually they will fall apart, into disorder.
How is this possible? Like all life, enzymes evolved by Darwinian natural selection. Once a primitive molecule acquired even very weak increase in activity, genetic mutations followed by selection, or more simply - a series of advantageous mistakes - did the rest. And it had a lot of time. But, here’s the catch - nothing evolves unless it already exists. Darwinian selection needs something to work on - some function to select for (or against). When life started more than three billion years ago, what created this “something” from randomness? It’s a big question.
With researchers from the Monash Biomedicine Discovery Institute (BDI), we have identified what we term ‘Structural Capacitance Elements’ in mutated proteins that are associated with many different types of human diseases, in particular a range of cancers.
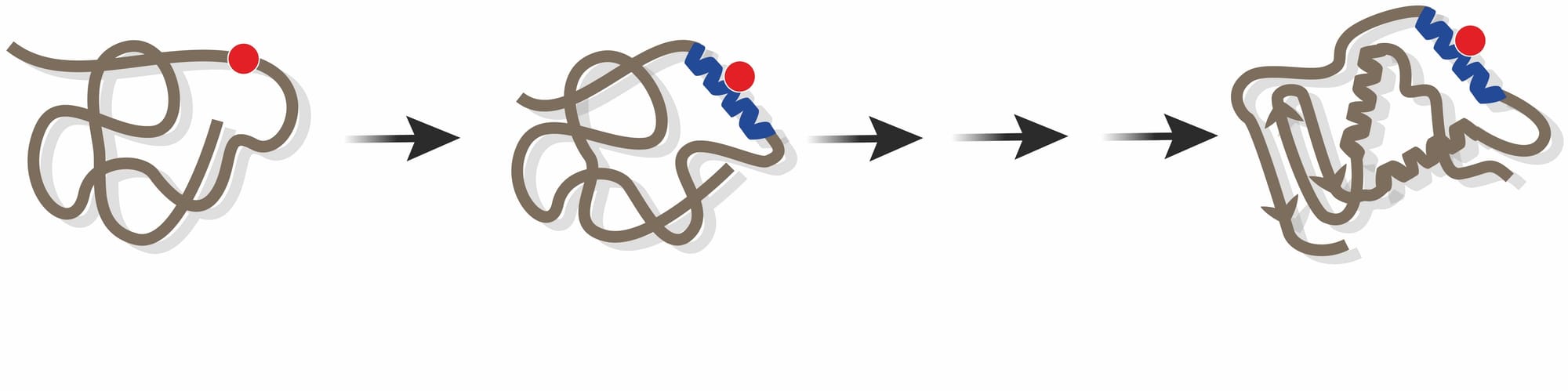
These elements are localised regions of disorder within proteins which retain the potential to change into ‘micro-structures’ following the introduction of a mutation. They act as seeds or ‘feedstock’ for evolution to proceed, providing the basis of an accelerated mechanism of Darwinian evolution by natural selection.
Our discovery has recently been published in the Journal of Molecular Biology. I was the lead researcher on this paper, and it is a significant discovery. Here’s why.
Until now, the accepted wisdom has been that mutations implicated in disease act by disrupting protein structure. The prime example of this idea is the tumour suppressor protein p53, where up to 40% of tumor-causing mutations in a wide range of cancers destabilise the protein structure, thus inactivating the protein. However, it is estimated that over 40% of human proteins contain ‘intrinsically disordered regions’ – meaning they have no well-defined structure – and many of these proteins are associated with human diseases, for example the kind of neurodegeneration seen in Alzheimer’s and Parkinson’s disease, and also cancer.
This prompted us to ask a very different question, and to turn the ‘loss-of-structure-function’ dogma on its head – are there disease-associated mutations in disordered regions of human proteins that could cause ordering?
We – myself and PhD student Chen Li, patiently worked our way through more than 68,000 human disease-associated mutations and were able to identify some. These regions of disorder within proteins have the potential to form an ordered structure following the introduction of a mutation. We realised that if single mutations in a gene could switch a previously disordered region into an ordered region, then this could potentially represent an entirely new and previously undiscovered mechanism by which Darwinian evolution elaborates on protein structures across rapid time scales.
Whereas the classic model of Darwinian evolution invokes a slow, gradual, and incremental mode of evolutionary change, if new pieces of protein structure could rapidly snap into place following the introduction of a single mutation, this may be a mechanism that facilitates rapid evolutionary change. Structure-inducing mutations, may, however, trigger inappropriate, “gain-of function” interactions that could result in human disease, suggesting that there is a ‘down side’ to employing this distinct new mechanism of evolutionary change.
We realise our work may have diverse implications. Not only does it shed light on the early evolution of protein structures, it suggests that disordered proteins are highly evolvable. One cannot easily re-sculpt Michelangelo’s David to Rodin’s “The Thinker.” It is better to start with a fresh block of marble.
Using this analogy, once a protein has evolved its specific, highly ordered shape, radical changes are not possible. Evolution of a new, totally different shape most likely starts from something a lot less ordered. The findings may also have implications for the identification and selective targeting of human disease. Understanding if and how a mutation may change the protein shape will be pivotal in targeting that protein for use in therapeutics that recognise the mutated region.
Read the full paper in the Journal of Molecular Biology titled Structural Capacitance in Protein Evolution and Human Diseases.
Dr Chen Li (now an NHMRC CJ Martin Fellow working at the ETH in Zurich) performed the work as part of his PhD in the Buckle lab, in collaboration with Dr Adrian Woolfson (Sangamo therapeutics).





