
As we move further into the 21st century, the world is becoming warmer and more populated. Global population density is estimated to grow by almost two billion people over the next few decades, the majority within India, Indonesia, and subsets of Sub-Saharan Africa.
For the most part, these regions exist within tropical and sub-tropical climates, causing individuals living within these areas to be exposed to substantial transmission of mosquito-borne infectious diseases.
Comprising the largest proportion of the global vector-borne disease burden, mosquito-borne infectious diseases are a major threat to public health.
Small but deadly
It seems counter-intuitive that the world’s deadliest animal is also one of the smallest. Adult mosquitos grow up to 1cm long and weigh only 2.5 milligrams, yet along with other disease vectors, cause about 700,000 deaths per year.
The Aedes, Anopheles, and Culex genera of mosquitoes transmit most disease-causing pathogens; however, a mere two mosquito species (Aedes aegypti and Aedes Albopictus) are responsible for transmission of chikungunya, dengue, yellow fever, and Zika alone.
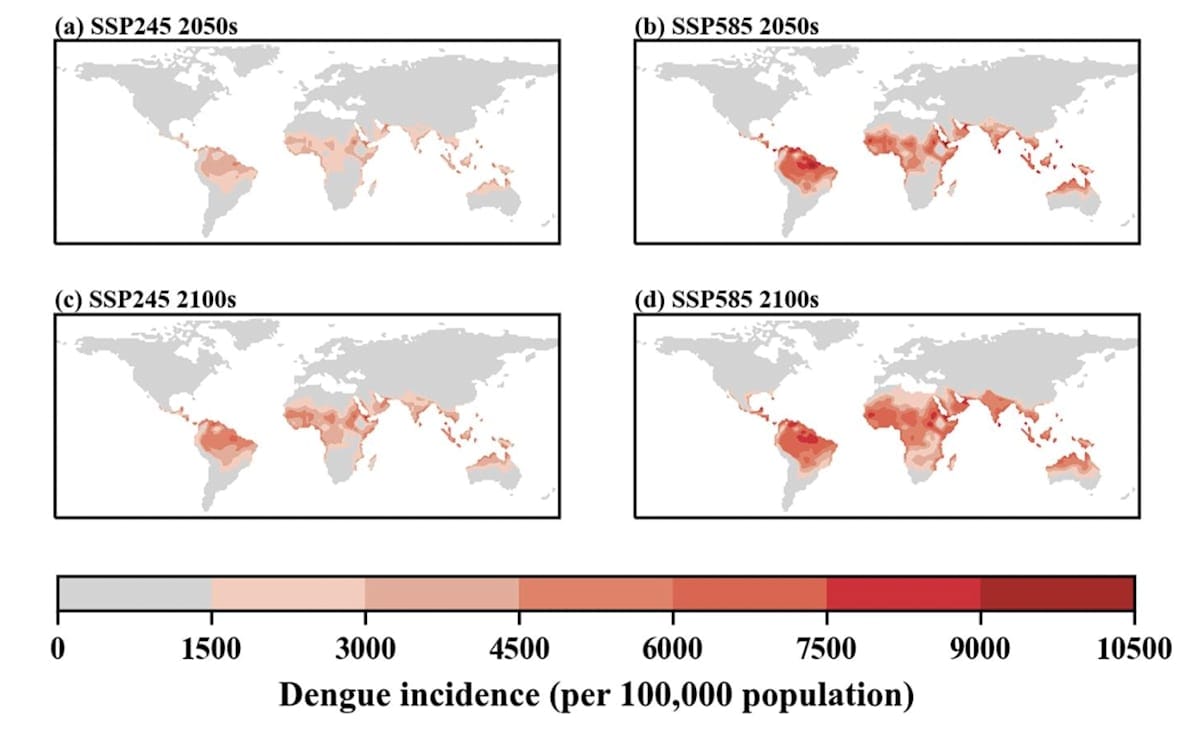
Presently, dengue and malaria pose the greatest significant public health threats borne by mosquitoes. Dengue transmission is increasing at an alarming rate, with 100-400 million new infections acquired per year – triple that of 1995.
Malaria, despite being one of the oldest diseases known to humans, claims the greatest morbidity and mortality of all vector-borne diseases.
However, progress in reducing the global malaria burden remains at a prolonged stall.
Mosquitoes are clearly formidable disease vectors, and several factors coalesce to make this so. Mosquitos are exceedingly adaptable to diverse environments; the dengue-transmitting Aedes albopictus mosquito species is highly invasive, with the capacity to breed independent of a tropical climate, allowing it to successfully form resident populations on every global continent except for Antarctica.
Frequent blood-feeding habits allow pathogens ingested by mosquitos to be distributed among humans at a rapid pace, and frequent reproduction ensures consistent mosquito circulation and disease transmission.
As cold-blooded ectotherms, mosquitos are temperature and climate change-sensitive. While temperature is a limiting factor for attributes of mosquito survival and transmission capacity, optimal temperature ranges are expanding, with features such as mosquito reproduction rate and viability having been found to improve along with temperature increases.
High relative humidity and rainfall are also known to enhance mosquito flight activity and host-seeking behaviour – both factors favouring higher transmission burdens.

Climate ties
In 2022, the International Panel for Climate Change concluded with high confidence that global temperature increases, as well as severe weather events, will contribute towards extending both the transmission season duration and spatial range for mosquito vectors of dengue and malaria.
A 2021 article published in The Lancet Planetary Health conducted multi-model, multi-scenario modelling to quantify the degree to which climate change will impact transmission season length and additional population at risk for these two diseases.
The authors found strong evidence for an increase in the epidemic belt for both dengue and malaria towards previously unaffected, temperate areas in Europe and North America.
Whereas malaria suitability was predicted to increase by 1.6 months in the Americas, the Eastern Mediterranean region and African highlands, dengue suitability was predicted to increase by four months in the Eastern Mediterranean and the Western Pacific lowlands.
Closer to home, transmission intensity of the most prevalent mosquito-borne infectious disease in Australia, Ross River virus (RRV), is increasing. While fatality is rare, RRV is a debilitating disease, with long-term disability a common outcome of infection.
Linkages have been found between RRV transmission within Australia and global warming, with epidemiological evidence showing temperature to be an important contributing factor.
Years lived with disability (YLD) after RRV infection were also associated with increasing temperatures, with 10.9 YLD per million population in the Northern Territory attributable to the increasing burden of RRV due to global warming.
Alongside increases in average global temperature, extreme weather events are occurring more frequently. A global analysis of lethal heat events projected that up to 74% of the world’s population in the year 2100 will endure extreme heat for almost one month per year, compared to 30% in 2017.
Droughts, which accompany extreme heat events, cause mosquitoes to gather around remaining water sources. Droughts have been determined as the main influence for increased West Nile fever epidemics, and are expected to increase the number of current cases by three-fold in the next 30 years.
On the other hand, flooding events will initially cause decreases in mosquito-borne infectious disease transmission, followed by epidemics as mosquitos find more suitable breeding sites.
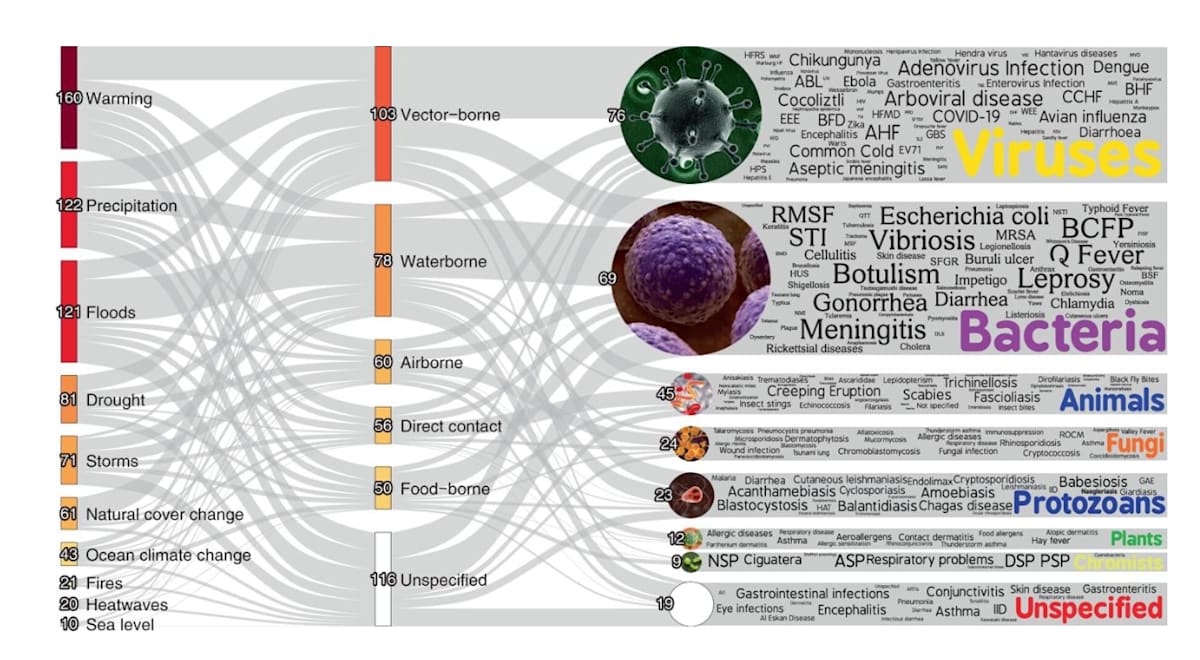
Vector-borne diseases comprise the greatest proportion of unique pathogenic diseases aggravated by climate change. Figure from Mora et al, 2022.
Global implications and future investment
Within the next five years, the global temperature is likely to exceed 1.5°C above pre-industrial levels, bringing us dangerously close to limits set within the Paris Agreement intended for decades, not years, from now. Predicted increases in populations at risk and climatic suitability for mosquito-borne infectious diseases will accompany these developments.
Spatio-temporal shifts in transmission patterns of mosquito-borne infectious diseases will likely result in outbreaks among populations without sufficient immunity, with potentially devastating consequences if public health infrastructure is unprepared.
Alongside the importance of a continued effort to limit increasing emissions, measures must be taken to reduce the impact of mosquito-borne infectious diseases.
Increased surveillance in high-transmission pockets to monitor case incidence, disease occurrence and immunity levels will be instrumental for reducing the public health burden of emergent diseases.
Initiatives such as the Monash University-led World Mosquito Program are furthering efforts to reduce transmission of chikungunya, dengue, yellow fever and Zika through the release of Wolbachia-infected Aedes aegypti mosquitoes.
Ultimately, continued investment in planning and research toward interventions geared to adapt to a warmer and more urbanised world are urgently required. These actions are critical for the support of health systems to control and prevent the incremental burden of these diseases worldwide.
Monash is pioneering a path to a greener, smarter, more equitable and sustainable future, where emissions are lower, and the natural environment and humans thrive. We look forward to participating at COP29, where we aim to accelerate global action on sustainability, empowering diverse voices from across the Indo-Pacific and influencing superior policy outcomes across a broad range of issues. Find out more monash.edu/cop29





