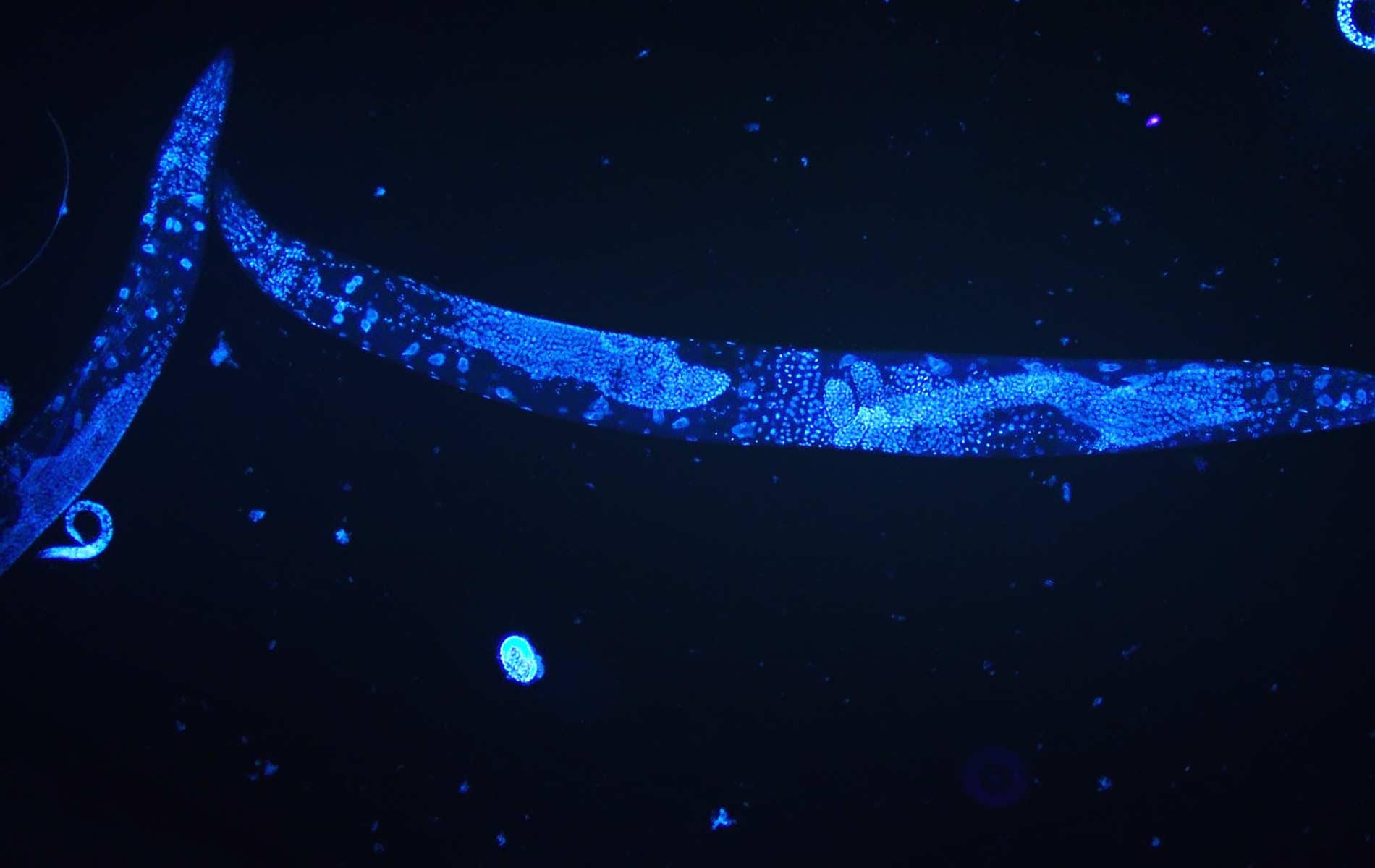
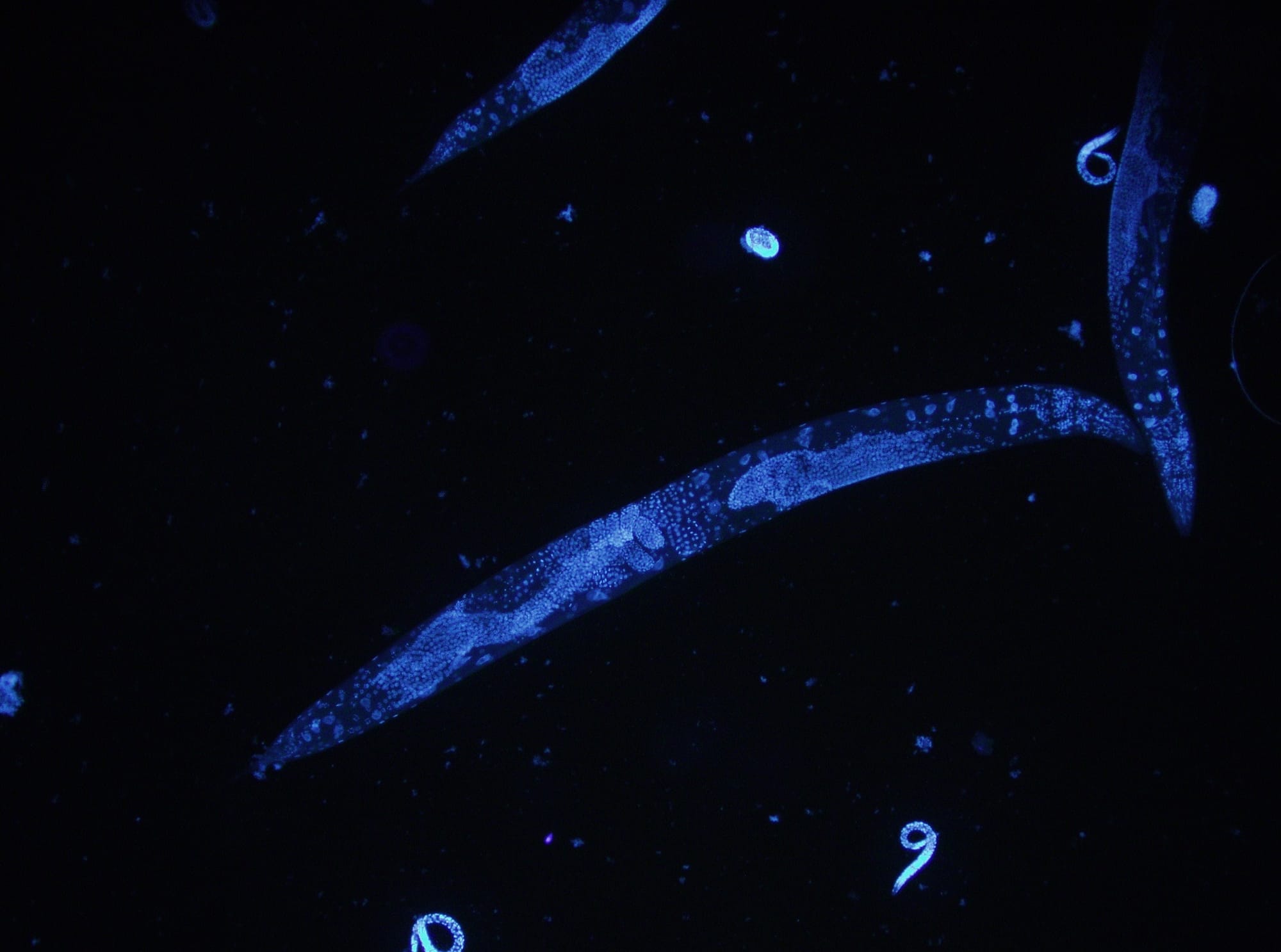
The roundworm Caenorhabditis elegans is a humble creature. It is only one millimetre long, lacks a respiratory and circulatory system, and is one of the simplest beings to have a nervous system. But this little worm holds secrets that can transform human health, and even one day help us understand how nerves can self-heal.
In 1998, C. elegans was the first multi-cellular organism to have its genome sequenced; in 1986 the first and only complete “neuronal wiring” map was made, based on the worm’s 302 neurons, and the 7000 connections between them.
“The worm is small, it’s inexpensive,” says Monash Associate Professor Roger Pocock. “We know more about this worm than any other organism on Earth.”
Biological investigations into C. elegans have so far been implicated in three Nobel prizes. The transparent little worm continues to be scrutinised by the most brilliant biologists on the planet.
Monash University has three separate worm labs. Dr Brent Neumann’s lab at the Biomedicine Discovery Institute (BDI) is investigating how worms with severed nerves self-heal – a process they hope will lead to human treatments for nerve injuries in the future.
The scientists from BDI, in collaboration with researchers from the Queensland Brain Institute at the University of Queensland, have found signals in the tiny transparent roundworm that control the mechanism by which severed nerves self-heal. Moreover, they have demonstrated how to control this process genetically, raising hopes for treating nerve injuries in humans in the future.
The discovery builds on the team’s landmark 2015 findings, in which the scientists discovered ‘axonal fusion’, a highly efficient yet simple repair process.
Professor Pocock’s lab has been examining the worm as a way of learning more about the function of genes in the human brain.
In 2015, Pocock received a $150,000 veski innovation fellowship that was matched in cash and kind by Monash University. As part of the fellowship, Pocock relocated his team of six from Copenhagen University to Monash.
The veski research has been focused on a gene called mir-234 in the brain of worms. The gene is directly related to the human gene mir-137, which is associated with schizophrenia – its role in human brain development is not understood.
Pocock’s worm lab research has now identified “a role for this gene in controlling communication between neurons”. The results will be published soon. The hope is that the research will eventually lead to schizophrenia treatments.
In February, the journal Proceedings of the National Academy of Sciences published research from Pocock’s lab that identified the gene responsible for regulating fat storage and metabolism in the worm.
“We found out that the gene functions in four neurons in the head that tells the intestine how much fat to store. Which is crazy, right? We weren’t looking for that,” Pocock says.
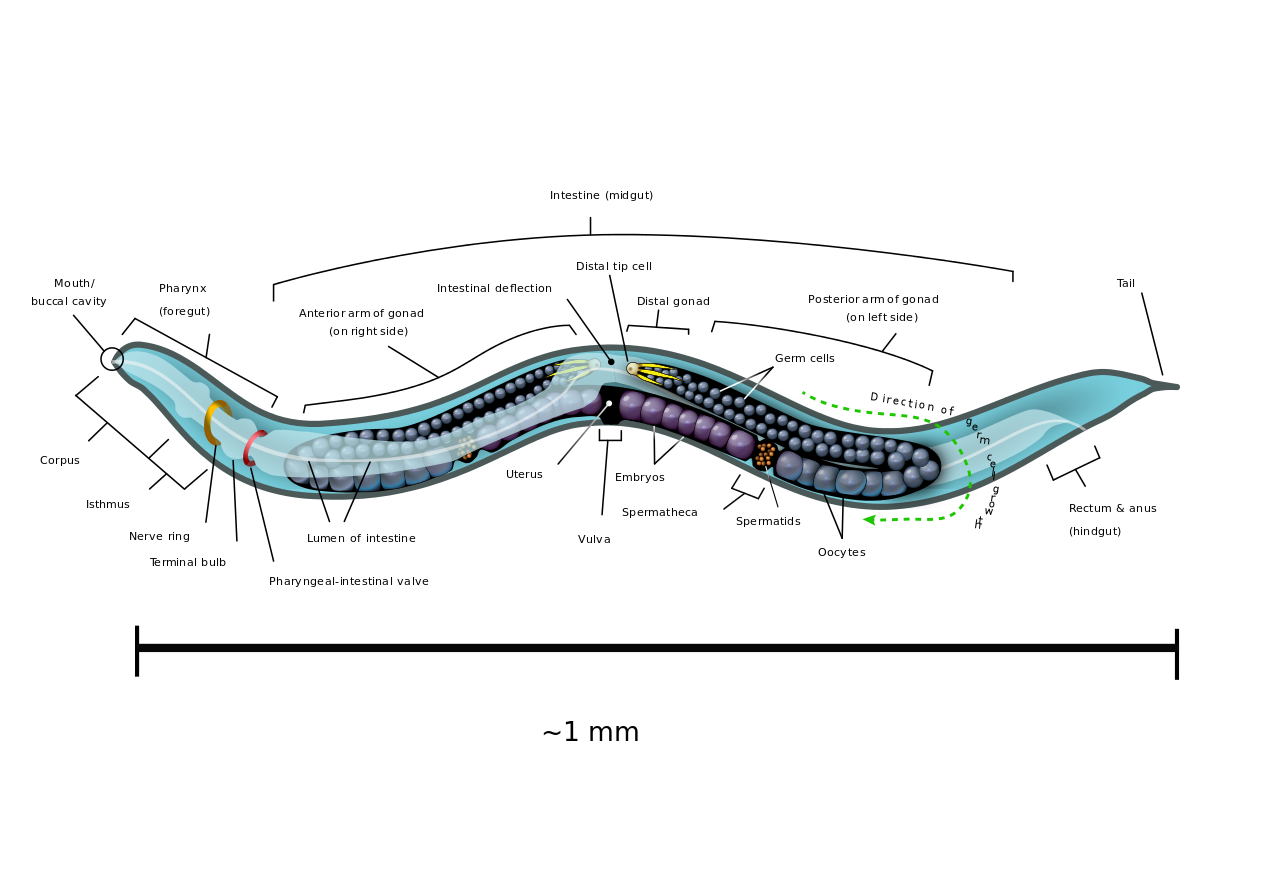
The researchers are now trying to discover how the worm’s brain communicates with its intestine – the answer could have implications for treating the human obesity epidemic down the track. To continue this work over the next five years, Pocock has been awarded a National Health and Medical Research Council senior research fellowship.
“The take-home from this study is that we were working on a gene and its function, and we found something new that you would never have guessed,” says Professor Pocock.
Pocock is a great advocate for pure biological research. He has been investigating C. elegans since he was a PhD student at the University of Oxford.
“We found out that the gene functions in four neurons in the head that tells the intestine how much fat to store. Which is crazy, right? We weren’t looking for that.”
The roundworm has already contributed to human understanding of programmed cell death (apoptosis), a finding that has proved important for understanding cancer. The first gene discovered that contributes to ageing, daf-2, was also found in the worm. Importantly for Pocock, the biologists who made these discoveries were willing to pursue their research for its own sake.
“That’s where major discoveries come from. From trying to understand biology. How does a gene work? How does a cell become a cell?”
Pocock’s initial research interest was cell fate – what mechanisms determine how and where cells develop. Biologists still don’t know.
“We are working on these sorts of questions now, and it’s a mystery still.”
His particular focus is neurobiology: “How do nerve cells become a certain type of nerve cell? Even in the worm that only has 302 neurons, there are a lot of different neurons that do different jobs.”
He expects he will be working on worms for the rest of his life. “Unexpected discoveries come all the time. That’s what I love.”





