Collagen, the most abundant protein in the body, is known for its role in healthy bones, skin, tendons, ligaments, connective tissues and muscles, including the heart. It makes up about one-third of all the protein in our bodies, and is often described as the “glue” that holds us together.
While there are 28 known types of collagen, it’s generally grouped into five main types:
- Type I is the most abundant, and is used to provide structure to skins, bones, tendons and ligaments.
- Type II is found in the cartilage that provides joint support, such as in your knees.
- Type III is found in muscles, arteries and organs.
- Type IV is found in the layers of your skin.
- Type V relates to your eyes, hair and skin.
As we age, though, our bodies produce less collagen, and the existing collagen can become abnormal or compromised. In some circumstances — such as cardiac disease, high blood pressure or heart inflammation — it can stiffen, rather than strengthen heart tissue, and the fine fibres are difficult to see until the scarring is advanced.
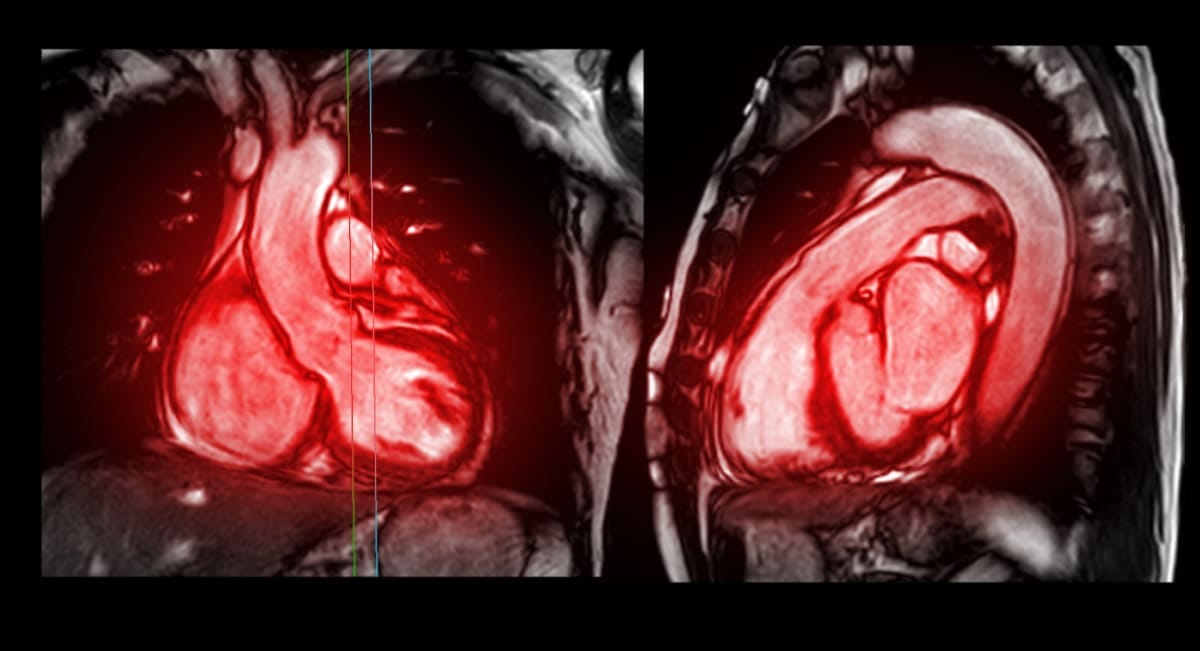
This can lead to a condition known as diffuse cardiac fibrosis, which is present in almost all chronic cardiac diseases. Detecting the abnormal collagen deposits, however, is difficult, and is usually done through a cardiac MRI or ultrasound scan.
Now, though, a team of Monash scientists has developed an imaging technique that can better-detect the abnormal collagen deposits in the heart, and which could lead to earlier diagnoses.
The Monash team at the School of Translational Medicine has developed a new radiotracer (a chemical probe used in positron emission tomography, or PET imaging) consisting of a short chain of amino acids called a T-peptide. Once injected into the body, the T-peptide accumulates where collagen has expanded, a sign that the heart muscle has become fibrotic.
Professor Christoph Hagemeyer, Dr Bianca Jupp and Dr Be’eri Niego, led by Dr Karen Alt, conducted the ground-breaking work, supported by Professor Paul Donnelly from Bio 21 at The University of Melbourne, who synthesised the tracer.
Their experimental study, funded by a Heart Foundation Vanguard Grant and published in the journal Radiology: Cardiothoracic Imaging, shows that in animal models, the method can more sensitively detect heart fibrosis than methods currently used in clinics – cardiac MRI and ultrasound scans.
First author Dr Be’eri Niego says diffuse cardiac fibrosis is challenging to diagnose.
“T-Peptide-based imaging enables us to directly see a build-up of collagen deposits throughout the heart muscle – something existing imaging doesn’t do very well.”
Dr Alt hopes human trials will confirm this as a new technique to identify people more likely to progress to serious disease, and be used for fibrotic diseases affecting other organs.
“T-peptide imaging is highly sensitive in picking up early stages of disease, when early intervention can have the most impact and can potentially reverse the disease,” she says.
“Some forms of diffuse cardiac fibrosis are actually reversible, so having more sensitive imaging will be important for early treatment of disease.”
Developing PET probes is just as challenging as developing a new drug. Internationally, more than 100 radiopharmaceuticals have been developed to date, but only a handful are approved for use in PET imaging.
One of the biggest challenges in the field is differentiation – developing a probe that is specific to an organ or disease stage.