
Since 2014, Monash researchers have tried to understand the mysterious molecular processes that occur during the first few days of human life.
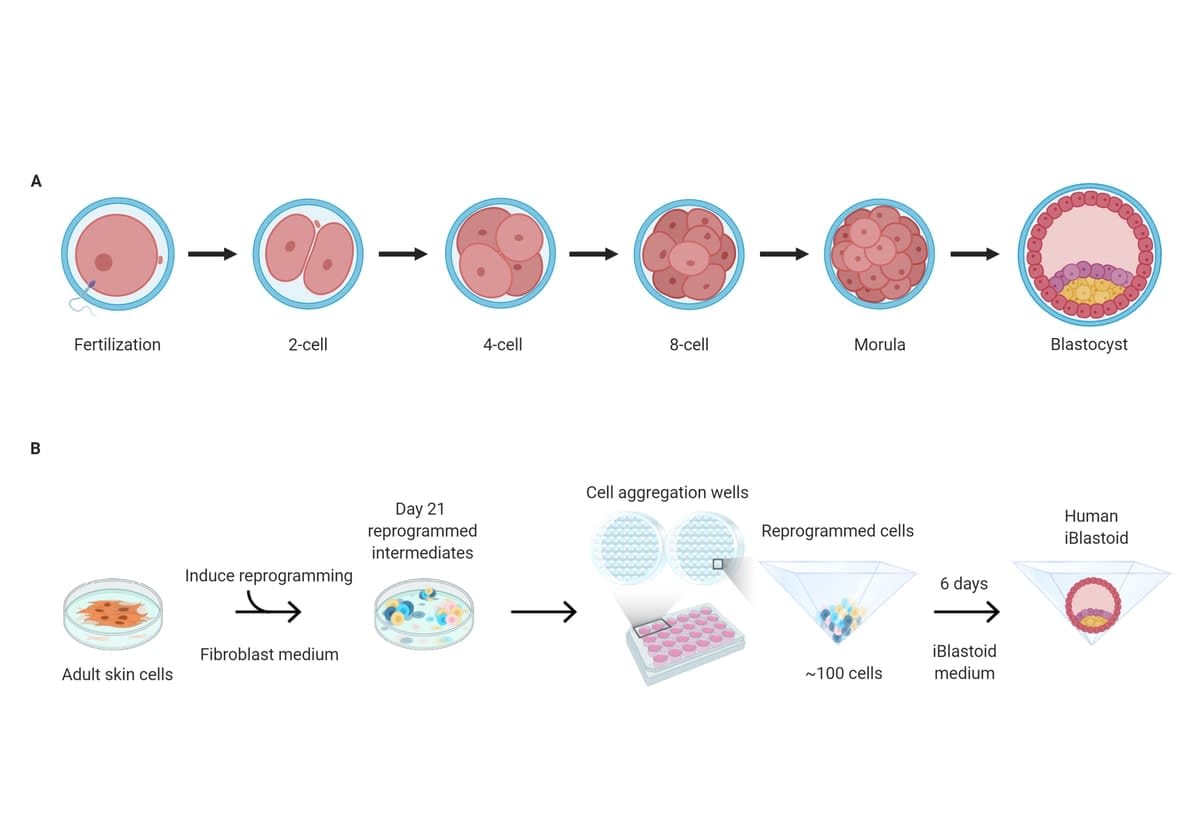
Late last year came the breakthrough. The University’s Polo Laboratory team unexpectedly produced a medical game-changer when it created what it calls an iBlastoid – the most accurate three-dimensional model of a blastocyst, or the cellular structure that becomes an early human embryo.
It’s made from skin cells and isn’t an embryo itself, so it can’t be used to make humans – to date, only a real embryo, made when sperm fertilises an egg, can do that. A “clone”, meanwhile, needs to begin with an unfertilised egg.
But an iBlastoid can be used for a short time to study specifically what happens in the first days after conception, in order to better examine infertility and congenital disease. The University has taken out a series of patents on the research.
“I hope iBlastoids will provide a great tool to study the problems without having to manipulate real embryos. We can also understand the reasons behind many congenital diseases.”
Professor Jose Polo
The research was conducted with Monash University human ethics approval, in compliance with Australian law and international guidelines.
These reference the “primitive streak rule”, or “day 14 rule”, that states that human blastocysts cannot be cultured beyond the development of the “primitive streak”, a structure that forms during the early stages of embryonic development.
This means the experiments were stopped in the lab between the equivalent days six and 10. The iBlastoid collapses if it’s left in the conditions in which it’s made, but can survive a few additional days.
Mitigating the embryo problem
Until now, embryos have only been studied with donated IVF embryos, which are hard to obtain, and “precious”, according to Professor Jose Polo, the Argentine-born lab leader from Monash’s Biomedicine Discovery Institute (BDI) and Australian Regenerative Medicine Institute (ARMI).
The Polo lab’s iBlastoid is a world-first invention, and mitigates the difficulties with experiments using real embryos. The lab’s medical scientists collaborated with peers from California, Singapore and Western Australia. Their findings are now detailed in Nature journal.
“Blastoids only allow us to study the very early stages of human development in the lab, so we can’t make a human in the lab,” says Professor Polo.
“It is a model of the initial steps of development, and is very different from cloning, because in cloning you must use an egg. Here we don’t use an egg, we use skin cells. It does not open any of those ‘cloning’ possibilities.”
According to the Director of ARMI, Professor Peter Currie, his “talented, relentless and passionate” colleague and his team are as rigorous about the ethics of genetic medicine as they are about the research.
“They’re steeped in the guidelines of what scientists can and cannot do,” he says. “This is very different to embryos – but in essence, the question being asked is, how does one cell become thousands and thousands of different cell types that we have in our body? And if we know how to do that, can we manipulate for therapeutic gain?”
It should open a pathway towards new therapies for infertility and genetic disease.
“Different reports indicate that at least 30% of all conceptions don’t pass the first 14 days – so this discovery may help researchers further understand the process,” Professor Polo says. “And it will allow, for the first time, the ability to study at scale what’s happening in the first days of development, and extend our knowledge and understanding of infertility and congenital diseases.
“In addition, as these iBlastoids are derived from skin cells, the source of cells could be patients that are having recurrent early pregnancy loss, so that the specific defect can be studied more closely.”
The path to the iBlastoid
The project began in 2014 through one of Professor Polo’s then PhD students, Xiaodong Liu, who was the first to develop a model of an early placenta from skin cells, which unexpectedly led to the iBlastoids.
The model could be considered an “organoid”, or lab-made (in-vitro) miniature copy of human organs such as parts of the brain, liver, kidneys and intestines.
“Organoids have allowed us to understand how the organs function, how they develop and assemble, and how different toxins or drugs affect them, and how disease affects them,” Professor Polo says. “But is the brain organoid a brain? No, it is not. This is the same for iBlastoids – they’re just a great model to study early development.”
Human blastocysts are devilishly tricky to study.
“They are so few, and have such small numbers of cells, less than 250, that they are difficult to physically or genetically study. The advantage of these iBlastoids, as opposed to human blastocysts resulting from egg-sperm fertilisation, is that a large number of genetically similar structures can be generated.”
Professor Polo and his team used a reprogramming technique that’s used to convert cell types in the lab.
Japanese researcher Shinya Yamanaka won the Nobel Prize in 2012 after using this technique in his research into induced pluripotent stem cells (iPS cells). These iPS cells can form any cell types in the body, and carry the enormous clinical promise of being able to “regenerate” failing organs and tissues in patients.
One of the huge potential benefits of an iBlastoid is that many hundreds or thousands can potentially be made, which can allow large-scale or mass-testing of the science surrounding fertility or congenital disease screening.
The unknowns of solving infertility
In terms of whether the invention can outright solve infertility, Professor Polo doesn’t know.
“Infertility is caused by many problems at different stages,” he says. “I hope iBlastoids will provide a great tool to study the problems without having to manipulate real embryos. We can also understand the reasons behind many congenital diseases.”
These might include cleft lip and palate, cerebral palsy, Down syndrome, spina bifida, cystic fibrosis, and congenital heart conditions.
“We understand what ethical issues we face in our fields. Scientists can only go as far as society will let them. Then we’re duty-bound to ask respectfully, ‘What do you want us to do now?’”
The radical invention brings the future of medicine several significant steps closer.
“Jose is always on the boundaries of this new science of what we call epigenetic reprogramming,” Professor Currie says. “He’s very interested in the first building blocks of life, and how cells commit to be different things in the embryo. It’s incredible science, fundamental science of the highest order.”
Professor Currie admits, however, that research on how babies are made – even if with a model such as an iBlastoid that could never make a baby – could be “confronting”.
“We understand what ethical issues we face in our fields,” he says. “Scientists can only go as far as society will let them. Then we’re duty-bound to ask respectfully, ‘What do you want us to do now?’"





