While the mention of the Fox3P+ T-regulatory cell – or ‘T-reg’ for short – would induce blank stares from most of the population, this ingenious and naturally occurring immune cell plays a vital role in human health.
Monash University’s Professor Jennifer Wilkinson-Berka and her team from the Department of Diabetes have found the cells can repair damaged blood vessels in the eye, and potentially save the eyesight of premature babies born with retina disease (retinopathy).
The team at Monash’s Central Clinical School were working on retinopathy caused by diabetes in adults, and approached the problem from the angle that this disease was identical to another type of retinopathy found in prematurely born babies. Their discovery could potentially save eyesight in both scenarios.
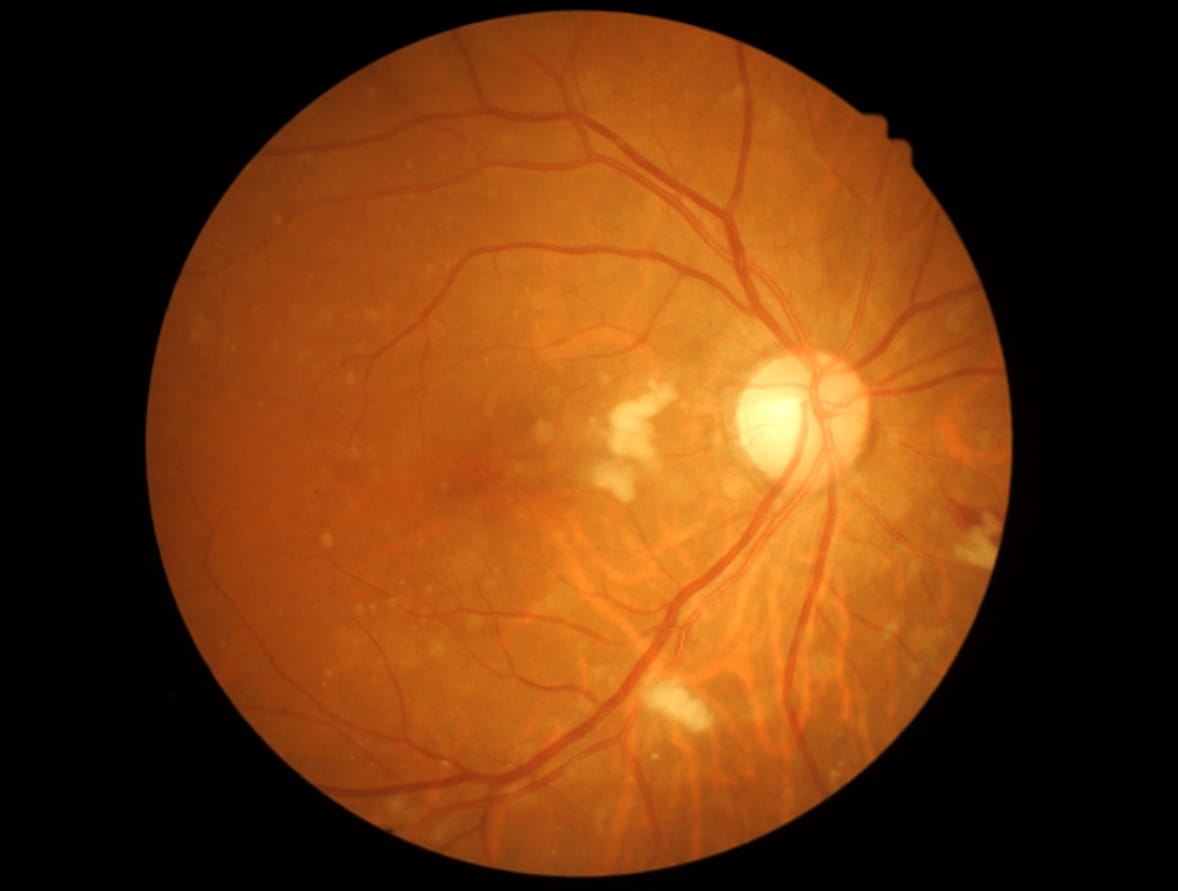
When born prematurely, babies require supplementary oxygen, but this life-saving measure can damage blood vessels within the retina if the concentration is too high. In that event, defective new blood vessels will form and leak, causing damage to the retina. This is called pathological angiogenesis.
The most common treatment for pathological angiogenesis is laser-eye surgery, in which a laser is used to burn away the damaged blood vessels in the eye. However, this comes with the risk of damaging the retina itself. Professor Wilkinson-Berka says more research is being done to apply the technique to the delicate anatomy of premature babies, but she’s hopeful a low-risk method can eventually be developed.
“There’s a certain immunotherapy where you can boost the number of T-regs, and it’s currently being evaluated in other diseases,” she said.
“But this may not be suitable for a very small baby – these are tiny babies. So, the next thing we want to be working on is a safe and well-tolerated T-reg therapy. We’re going to be obtaining T-regs from premature babies and looking at the molecular signature of T-regs and seeing if there’s something unusual about them. We’ll be doing this work with the Royal Children’s Hospital in Melbourne.”
Many premature babies today still grow up with at least partial blindness, especially in Third World countries where hospitals struggle to provide optimal treatment.
As technology in First and Third World countries improves, more premature babies will be saved by oxygen and, as a consequence, in the coming decades we’ll see a rise in retinopathy, Professor Wilkinson-Berka warns.
The research focus, however, is on retinopathy caused by diabetes in adults.
Diabetes is poised to be a massive public health problem in the coming years. In First World countries, the consumption of foods high in sugar, combined with more sedentary lifestyles, has led to a dramatic rise in obesity, which greatly elevates a person’s risk of developing type-2 diabetes. It’s estimated that by 2040, about 642 million people worldwide will suffer from the disease.
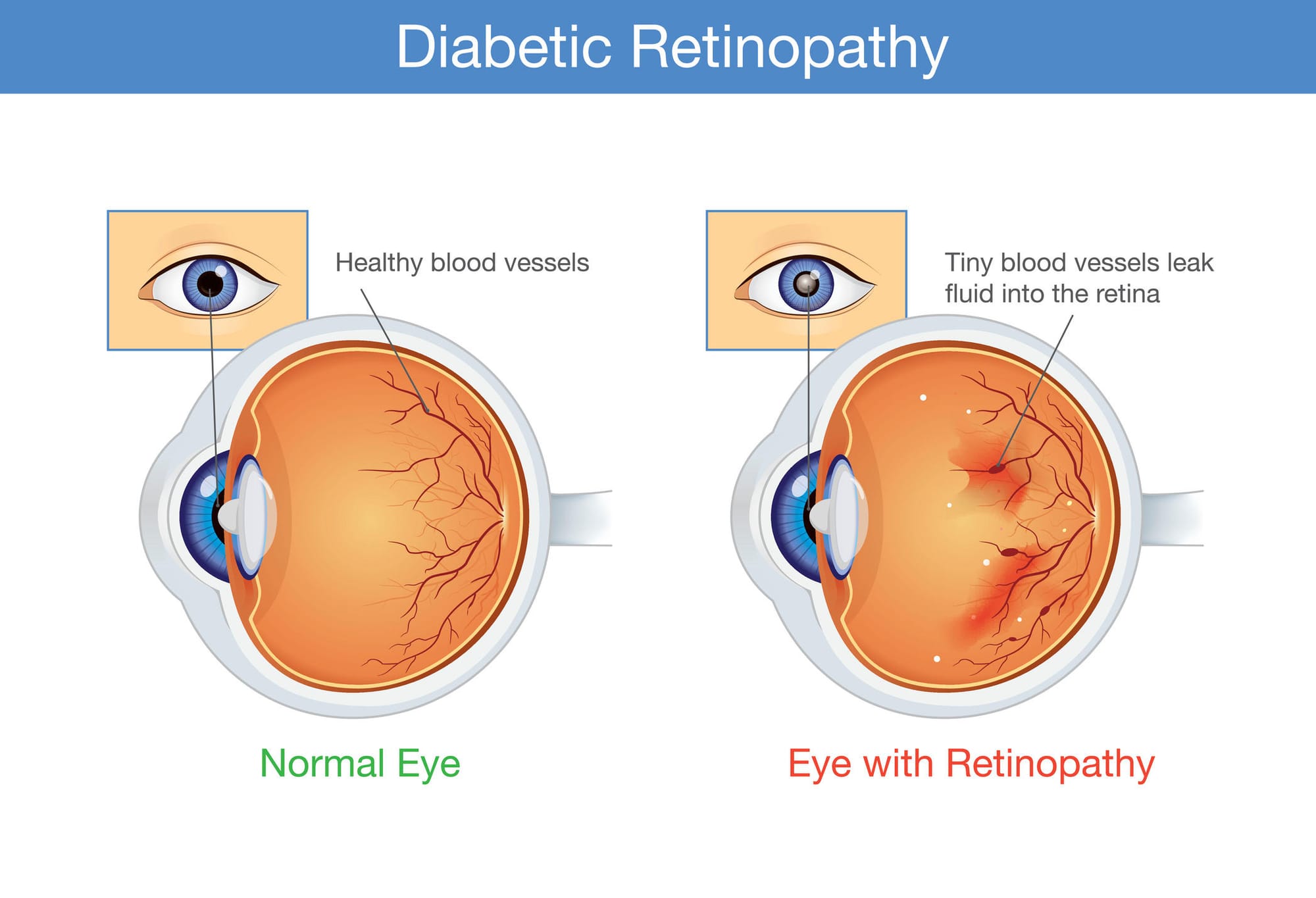
Among the diabetes’ added complications is blindness caused by retinopathy. This occurs because inflammation and other factors that influence blood vessels in the eye leads to pathological angiogenesis, with those new blood vessels branching out from old like the branches on a tree, but defective and leaking blood and other liquid into the retina, potentially causing permanent damage.
Which is where the Fox3P+ T-regulatory cell – T-reg – comes in.
Professor Wilkinson-Berka’s team found that T-regs moved to the retina blood vessels when a person had pathological angiogenesis, to try to repair the damage caused by hypertension and inflammation. But they found the natural strength and number of these T-regs was too low to adequately prevent and repair diabetic retinopathy and retinopathy of prematurity. However, by increasing the number and strength of the T-regs through medication, the cells gain the capacity they need to repair the damage.
The advantage of this discovery as an option for treatment is that it may reduce the risk of side effects when compared to laser surgery. It can also be used as a preventative measure against pathological angiogenesis, as the T-regs can also deactivate the inflammatory cells that cause inflammation and hypertension in the retina blood vessels.
Professor Wilkinson-Berka says the discovery can be attributed to the combining of widely varied skill sets that helps push innovative thinking at Monash.
“You have to be interested in lots of different things today to do good science. In my day you did your physiology, you did your anatomy, you did your biochemistry – you stayed in those intellectual silos. But today you need to move in between those silos.”
She came to Monash as an anatomist from the University of Melbourne with an interest in hypertension and kidney disease. After changing her university, her department and her research focus, she found that her background – unusual for someone researching retinopathy – proved to be an advantage, as she could tackle the problem from an innovative angle.
“That’s the great strength of Monash,” she says. “I believe because it’s invested in platform technologies, proteomics, all the biology, imaging, the synchrotron – you’ve got all the things that come together that makes it really powerful, and investing in those platforms is important. And the bringing together of different departments is also important.”





